.

Tar sandstone from California, United States.

Bituminous outcrop of the Puy de la Poix, Clermont-Ferrand, France.
The sources of unconventional oil[]
What are oil sands[]
Oil sands, tar sands or, more technically, bituminous sands, are a type of unconventional petroleum deposit.
Oil sands are either loose sands or partially consolidated sandstone containing a naturally occurring mixture of sand, clay, and water, saturated with a dense and extremely viscous form of petroleum technically referred to as bitumen (or colloquially as tar due to its superficially similar appearance). Natural bitumen deposits are reported in many countries, but in particular are found in extremely large quantities in Canada. Other large reserves are located in Kazakhstan and Russia. The estimated worldwide deposits of oil are more than 2 trillion barrels (320 billion cubic metres); the estimates include deposits that have not been discovered. Proven reserves of bitumen contain approximately 100 billion barrels, and total natural bitumen reserves are estimated at 249.67 Gbbl (39.694×109 m3) worldwide, of which 176.8 Gbbl (28.11×109 m3), or 70.8%, are in Alberta, Canada.
Oil sands reserves have only recently been considered to be part of the world's oil reserves, as higher oil prices and new technology enable profitable extraction and processing. Oil produced from bitumen sands is often referred to as unconventional oil or crude bitumen, to distinguish it from liquid hydrocarbons produced from traditional oil wells.
The crude bitumen contained in the Canadian oil sands is described by the National Energy Board of Canada as "a highly viscous mixture of hydrocarbons heavier than pentanes which, in its natural state, is not usually recoverable at a commercial rate through a well because it is too thick to flow." Crude bitumen is a thick, sticky form of crude oil, so heavy and viscous (thick) that it will not flow unless heated or diluted with lighter hydrocarbons such as light crude oil or natural-gas condensate. At room temperature, it is much like cold molasses. The World Energy Council (WEC) defines natural bitumen as "oil having a viscosity greater than 10,000 centipoise under reservoir conditions and an API gravity of less than 10° API". The Orinoco Belt in Venezuela is sometimes described as oil sands, but these deposits are non-bituminous, falling instead into the category of heavy or extra-heavy oil due to their lower viscosity. Natural bitumen and extra-heavy oil differ in the degree by which they have been degraded from the original conventional oils by bacteria. According to the WEC, extra-heavy oil has "a gravity of less than 10° API and a reservoir viscosity of no more than 10,000 centipoise".
According to the study ordered by the Government of Alberta and conducted by Jacobs Engineering Group, emissions from oil-sand crude are 12% higher than from conventional oil.
What are oil shales[]

Outcrop of Ordovician oil shale (kukersite), northern Estonia.
Oil shale, also known as kerogen shale, is an organic-rich fine-grained sedimentary rock containing kerogen (a solid mixture of organic chemical compounds) from which liquid hydrocarbons called shale oil (not to be confused with tight oil—crude oil occurring naturally in shales) can be produced. Shale oil is a substitute for conventional crude oil; however, extracting shale oil from oil shale is more costly than the production of conventional crude oil both financially and in terms of its environmental impact. Deposits of oil shale occur around the world, including major deposits in the United States. Estimates of global deposits range from 4.8 to 5 trillion barrels (760×109 to 790×109 m3) of oil in place.
Heating oil shale to a sufficiently high temperature causes the chemical process of pyrolysis to yield a vapor. Upon cooling the vapor, the liquid shale oil—an unconventional oil—is separated from combustible oil-shale gas (the term shale gas can also refer to gas occurring naturally in shales). Oil shale can also be burned directly in furnaces as a low-grade fuel for power generation and district heating or used as a raw material in chemical and construction-materials processing.
Oil shale gains attention as a potential abundant source of oil whenever the price of crude oil rises. At the same time, oil-shale mining and processing raise a number of environmental concerns, such as land use, waste disposal, water use, waste-water management, greenhouse-gas emissions and air pollution. Estonia and China have well-established oil shale industries, and Brazil, Germany, and Russia also utilise oil shale.
General composition of oil shales constitutes inorganic matrix, bitumens, and kerogen. Oil shales differ from oil-bearing shales, shale deposits that contain petroleum (tight oil) that is sometimes produced from drilled wells. Examples of oil-bearing shales are the Bakken Formation, Pierre Shale, Niobrara Formation, and Eagle Ford Formation.
Oil shale, an organic-rich sedimentary rock, belongs to the group of sapropel fuels. It does not have a definite geological definition nor a specific chemical formula, and its seams do not always have discrete boundaries. Oil shales vary considerably in their mineral content, chemical composition, age, type of kerogen, and depositional history and not all oil shales would necessarily be classified as shales in the strict sense. According to the petrologist Adrian C. Hutton of the University of Wollongong, oil shales are not "geological nor geochemically distinctive rock but rather 'economic' term." Their common feature is low solubility in low-boiling organic solvents and generation of liquid organic products on thermal decomposition.
Oil shale differs from bitumen-impregnated rocks (oil sands and petroleum reservoir rocks), humic coals and carbonaceous shale. While oil sands do originate from the biodegradation of oil, heat and pressure have not (yet) transformed the kerogen in oil shale into petroleum, that means that its maturation does not exceed early mesocatagenetic.
General composition of oil shales constitutes inorganic matrix, bitumens, and kerogen. While the bitumen portion of oil shales is soluble in carbon disulfide, kerogen portion is insoluble in carbon disulfide and can contain iron, vanadium, nickel, molybdenum, and uranium. Oil shale contains a lower percentage of organic matter than coal. In commercial grades of oil shale the ratio of organic matter to mineral matter lies approximately between 0.75:5 and 1.5:5. At the same time, the organic matter in oil shale has an atomic ratio of hydrogen to carbon (H/C) approximately 1.2 to 1.8 times lower than for crude oil and about 1.5 to 3 times higher than for coals. The organic components of oil shale derive from a variety of organisms, such as the remains of algae, spores, pollen, plant cuticles and corky fragments of herbaceous and woody plants, and cellular debris from other aquatic and land plants. Some deposits contain significant fossils; Germany's Messel Pit has the status of a Unesco World Heritage Site. The mineral matter in oil shale includes various fine-grained silicates and carbonates. Inorganic matrix can contain quartz, feldspars, clays (mainly illite and chlorite), carbonates (calcite and dolomites), pyrite and some other minerals.
Geologists can classify oil shales on the basis of their composition as carbonate-rich shales, siliceous shales, or cannel shales.
Another classification, known as the van Krevelen diagram, assigns kerogen types, depending on the hydrogen, carbon, and oxygen content of oil shales' original organic matter. The most commonly used classification of oil shales, developed between 1987 and 1991 by Adrian C. Hutton, adapts petrographic terms from coal terminology. This classification designates oil shales as terrestrial, lacustrine (lake-bottom-deposited), or marine (ocean bottom-deposited), based on the environment of the initial biomass deposit. Known oil shales are predominantly aquatic (marine, lacustrine) origin. Hutton's classification scheme has proven useful in estimating the yield and composition of the extracted oil.
Some in both Israel and Palestine have regarded it on occasion as a possible energy source during the late 1970s and early 1990s. The USSR, Brazil, China, the GDR, the FRG, Russia and Estonia regard it as a handy local source of oil that can help maintain ther energy industry.
What is Gilsonite/bitumen stone[]
Formation and compensation[]
Gilsonite, also known as uintahite or asphaltum, is a bitumen-impregnated rock (asphaltite) mainly found in the Uintah Basin of Utah and Colorado, United States. It is a naturally occurring solid hydrocarbon bitumen. Although it occurs also in other locations, its large-scale production occurs only in the Uintah Basin.
The mineral Gilsonite is categorized as a solvable material in oil solutions such as CS2 or TCE (Trichloroethylene). Major component of Gilsonite is carbon, while it contains several other elements including Nitrogen and Sulphur and some volatile matters.
Gilsonite is mined in underground shafts and resembles shiny black obsidian. Discovered in the 1860s, it was first marketed as a lacquer, electrical insulator, and waterproofing compound about twenty-five years later by Samuel H. Gilson.
Usage, location and formation[]
By 1888 Gilson had started a company to mine the substance, but soon discovered the vein was located on the Uintah and Ouray Indian Reservation. Under great political pressure Congress removed some 7,000 acres (28 km2) from the reservation on May 24, 1888 to allow the mining to proceed legally. Gilsonite mining became the first large commercial enterprise in the Uintah Basin, causing most of its early population growth.
Natural Bitumen (Gilsonite) reserves are spread over the globe in quite a lot of regions especially within basins. With regards to the geological nature of each area, natural bitumen can be found in different forms.
This unique mineral is used in more than 160 products, primarily in dark-colored printing inks and paints, oil well drilling muds and cements, asphalt modifiers, foundry sand additives, and a wide variety of chemical products. The trademark, registered in 1921, belongs to the American Gilsonite Company.
Mining Gilsonite during World War II was by hand, using a six-pound pick and then shoveling the ore into 200 pound sacks, which were sewn by hand. In 1949 at the Parriette Gilsonite mine near Myton, Utah, Reed Smoot McConkie set the world record for ore mined by hand. Using his pick and shovel, he mined 175 bags of ore in an 8-hour day, 950 bags in a six-day week, 1925 bags in a month and 15,000 bags in one year.
Gilsonite-brand uintahite's earliest applications included paints for buggies and emulsions for beer-vat lining. It was used by Ford Motor Company as a principal component of the japan black lacquer used on most of the Ford Model T cars.
Gilsonite is one of the key ingredients in Minwax wood stain.
Firms like Zista.co mine the reserve in Uintah Basin of Utah and Colorado. Small, but economically unenviable amounts are also found in Wyoming, Nevada, California, Oregon and Palestine.
What is cannel coal[]

Cannel coal from N.E. Ohio.
Cannel coal or candle coal, is a type of bituminous coal, also classified as terrestrial type oil shale. Due to its physical morphology and low mineral content cannel coal is considered to be coal but by its texture and composition of the organic matter it is considered to be oil shale. Although historically the term cannel coal has been used interchangeably with boghead coal, a more recent classification system restricts cannel coal to terrestrial origin, and boghead coal to lacustrine environments.
History[]
In England a member of the Bradshaigh family discovered a plentiful shallow seam of smooth, hard, cannel coal on his estate, in Haigh, Greater Manchester in the 16th century. The shallow depth at which it was found meant it was suitable for the simple surface mining methods available at that time. It could be worked and carved, and was prized for fireplaces as an excellent fuel that burned with a bright flame, was easily lit, and left virtually no ash.
Cannel coal commanded a premium price as a grate fuel for use in home fireplaces. It burned longer than wood, and had a clean, bright flame. It is more compact and duller than ordinary coal, and can be worked in the lathe and polished. In the Durham coalfield and elsewhere carving cannel coal into ornaments was a popular pastime amongst the miners.
The excess of hydrogen in a coal, above the amount necessary to combine with its oxygen to form water, is known as disposable hydrogen, and is a measure of the fitness of the coal for use in the manufacture of coal gas. Such coal, although of very small value as fuel, commands a specially high price for gas-making. Cannel coal was used as a major feedstock for the historical manufactured gas industry, as the gas produced from it was valuable for lighting due to the luminosity of the flame it produced. Cannel gas was widely used for domestic lighting throughout the 19th century before the invention of the incandescent gas mantle by Carl Auer von Welsbach in the 1880s. Following the introduction of the gas mantle, cannel coal lost favour as a manufactured gas feedstock as the gas mantle could produce large quantities of light without regard for the flame luminosity of the gas burnt.
On October 17, 1850, James Young, of Glasgow, Scotland, patented a method for the extraction of paraffin from cannel coal. It was widely used from 1850 to 1860 in the manufacture of coal oil, which today would be called shale oil. The principal consumer product was the illuminating oil kerosene. In 1860, there were 55 companies in the United States making coal oil from cannel coal, most of them near the cannel coal mines, in New York, Pennsylvania, Ohio, Kentucky, and western Virginia (now West Virginia). The discovery of petroleum deposits in the US, starting with the Drake Oil Well in 1859, made petroleum a cheaper raw material for making kerosene and drove the American oil shale industry out of business.
Chemical and physical composition[]
Cannel coal is brown to black oil shale. It comes from resins, spores, waxes, and cutinaceous and corky materials of terrestrial vascular plants, in part from Lycopsid (scale tree). Cannel coal was accumulated in ponds and shallow lakes in peat-forming swamps and bogs of the Carboniferous age under oxygen-deficient conditions. Thus cannel coal seams are shallow and often found above other deposits, while the coal itself, being rich in oils, burns long, with a bright yellow flame and little ash. The modern Lycopodiopsida relatives of these lycopsids (scale trees), with their similar high oil content, high surface area spores, are the source of highly flammable lycopodium powder.
Cannel coal is also lower in fixed carbon than typical bituminous coal. It includes various amounts of vitrinite and inertinite. Analytically, cannel coal consists of micrinites, macerals of the exinite group, and certain inorganic materials.
Coal oil is a shale oil obtained from the destructive distillation of cannel coal, mineral wax, or bituminous shale, once used widely for illumination.
Chemically similar to the more refined, petroleum-derived kerosene, it consists mainly of several hydrocarbons of the alkane series, with 10 to 16 carbon atoms in each molecule and a higher boiling point (175–325 °C) than gasoline or the petroleum ethers and lower than the oils.
The term was in use by the late 18th century, for oil produced as a byproduct of the production of coal gas and coal tar. In the early 19th century it was discovered that coal oil distilled from cannel coal could be used in lamps as an illuminant, although the early coal oil burned with a smokey flame, so that it was used only for outdoor lamps; cleaner-burning whale oil was used in indoor lamps.
Usages[]
Coal oil that burned cleanly enough to compete with whale oil as an indoor illuminant was first produced in 1850 by James Young on the Union Canal in Scotland, who patented the process. Production thrived in Scotland, creating much wealth for Young.
In the United States, coal oil was widely manufactured in the 1850s under the trade name Kerosene, manufactured by a process invented by Canadian geologist Abraham Gesner. Young triumphed in his patent lawsuit against the Gesner process in the United States in 1860. But by that time, US coal oil distillers were switching over to refining cheaper petroleum, after the discovery of abundant petroleum in western Pennsylvania in 1859, and oil from coal operations ceased in the US.
Because kerosene was first derived from cannel coal, classified as terrestrial type of oil shale, it continued to be popularly referred to as "coal oil" even after production shifted to petroleum as a feedstock. Technically, refined hydrocarbons of the alkane series with 10 to 16 carbon atoms are the same thing whether taken from coal or petroleum.
Coal oil has been used to provide lighting. Coal oil was once used as an internal and topical home remedy as a general cure-all for many ailments, including coughs, flu, cuts, abrasions, and wounds. Internal applications were administered by adding this toxic petroleum product to sugar cubes, molasses, honey or some other substance to mask the taste, while topical applications were applied adding to bandages or by pouring the coal oil directly on the affected area.
What is Ampelite\Ampélite[]
Ampelite\Ampélite, in natural history, is a black, bituminous substance that dissolves in oil; perhaps cannel coal. Historically, it was used to blacken eyebrows and hair. It mostly occers in France and nighboring parts of Germany, Switzerland, Spain, Andorra, Luxembourg and Belgium.
What is Lamosite[]
Formation[]
Lamosite is an olive-gray brown or dark gray to brownish black lacustrine-type oil shale, in which the chief organic constituent is lamalginite derived from lacustrine planktonic algae. In minor scale it also consists of vitrinite, inertinite, telalginite, and bitumen.
Location[]
Lamosite deposits are the most abundant and largest oil shale deposits beside of marinite deposits. The largest lacustrine-type oil shale deposits are the Green River Formation in western United States, a number deposits in eastern Queensland, Australia, and the New Brunswick Albert Formation and several other deposits in Canada.
What is Marinite[]
Formation[]
Marinite is a gray to dark-gray or black oil shale of marine origin in which the chief organic components are lamalginite and bituminite derived from marine phytoplankton, with varied admixtures of bitumen, telalginite and vitrinite. Marinite deposits are the most abundant oil-shale deposits. They are generally widespread but at the same time they are relatively thin and often of restricted economic importance. Typical environments for marinite deposits are found in epeiric seas (e.g. on broad shallow marine shelves or below inland seas where wave action is restricted and currents are minimal).
Location[]
The largest marinite-type oil-shale deposits are the Devonian–Mississippian oil-shales deposits in eastern United States. In Canada, the marinite-type of oil-shale deposits include the Devonian Kettle Point Formation and the Ordovician Collingwood Shale of southern Ontario, the Cretaceous Boyne and Favel deposits in the Prairie Provinces of Manitoba, Saskatchewan, and Alberta, and the Anderson Plain and the Mackenzie Delta deposits in the Northwest Territories.
Outside North America, marinite occurs in the Irati Formation in Brazil, deposits in the Middle East and North Africa, and in Sweden. Some plans had been set out for it's use in Sweden during the mid to late 1950s, but useing nucliar power and the continued use of hydro-power were chosen on ecanomic grounds.
What is Tasmanite?[]
Tasmanite is a rock type almost entirely consisting of the prasinophyte alga Tasmanites. It is commonly associated with high-latitude, nutrient-rich, marginal marine settings find in Tasmania. It is classified as marine type oil shale. It is found in many oil-prone source rocks and, when present, contributes to the oil generation potential of the rock. Some sources also produce a red-brown translucent material similar to amber which has also been called tasmanite.
It is mostly located in Tasmania with other smaller outcrops in NZ, PNG and mainland Australia.
What is Montan wax?[]
Montan wax, also known as lignite wax or OP wax, is a hard wax obtained by solvent extraction of certain types of lignite or brown coal. Commercially viable deposits exist in only a few locations, including Amsdorf, Germany, and in the Ione Basin, near Ione, California.
Its color ranges from dark brown to light yellow when crude, or white when refined. Its composition is non-glyceride long-chain (C24–C30) carboxylic acid esters (62–68 weight %), free long-chain organic acids (22–26%), long-chain alcohols, ketones, and hydrocarbons (7–15%), and resins; it is in effect a fossilized plant wax. Its melting range is betwwen 82–95 °C.
It is used for making car and shoe polishes, paints, and phonograph records, and as lubricant for molding paper and plastics. About a third of total world production is used in car polish. Formerly, its main use was making carbon paper. Unrefined montan wax contains asphalt and resins, which can be removed by refining. Montan wax in polishes improves scuff resistance, increases water repellence, and imparts high gloss.
What is Kukersite?[]
Kukersite is a light-brown marine type oil shale of Ordovician age. It is found in the Baltic Oil Shale Basin in Estonia and North-West Russia. It is of the lowest Upper Ordovician formation, formed some 460 million years ago. It was named after the German name of the Kukruse Manor in the north-east of Estonia by the Russian paleobotanist Mikhail Zalessky in 1917.
The Baltic Oil Shale Basin covers about 3,000 to 5,000 square kilometres (1,200 to 1,900 sq mi). Main kukersite deposits are Estonian and Tapa deposits in Estonia, and Leningrad deposit in Russia (also known as Gdov or Oudova deposit). Other occurrences in Russia are Veimarn and Chudovo–Babinskoe deposits. The Estonian deposit, which covers about 2,000 square kilometres (770 sq mi), is exploited industrially; the Tapa deposit is not accounted as reserves due its lower value which makes its extraction economically inexpedient. The Leningrad deposit was exploited industrially but operations have ceased.
Some minor kukersite resources occur in sedimentary basins of Michigan, Illinois, Wisconsin, North Dakota, and Oklahoma in North America and in Amadeus and Canning basins Australia.
Geology[]
Kukersite occurs within the Kukruse and Uhaku stages of the Viivikonna and Kõrgekallas formations, as an often calcareous layer. In northern Estonia there are a total of 50 oil shale layers of kukersite, of which six lowest form a 2.5-to-3-metre (8 ft 2 in to 9 ft 10 in) thick mineable bed. In this part kukersite lies near the surface while southward and westward its depth increases and its thickness and quality decreases.
Estonia's kukersite represents about 1.1% of global and 17% of European oil shale resources. The total kukersite resources in Estonia are estimated to be about 4.8 billion tonnes, including about 1 billion tonnes economically proven reserve, 0.3 billion tonnes economic probable reserve and about 3.5 billion tonnes uneconomical proven and probable reserve. Economically proven and probable reserves forms active resource, which is defined as mineable deposits with energy ratings of at least 35 gigajoules per square metre and calorific values of at least 8 MJ/kg, located in areas without environmental restrictions. Energy rating of the oil shale mining block is calculated as the sum of the products of thickness, calorific values and densities of all oil shale layers and limestone interlayers. Up to 50% of active resources are designated as recoverable.
The Leningrad deposit consists of 3.6 billion tonnes of kukersite, including more than one billion tonnes of economically proven and probable reserves.
Composition[]
Fossils (various bryozoans) in Ordovician period kukersite oil shale, northern Estonia Estonian kukersite deposits are one of the world's highest-grade deposits with organic content varying from 15% to 55% with average more than 40%, and it has 65–67% conversion ratio into shale oil and oil shale gas. Fischer Assay oil yield is 30 to 47%.] Its organic matter has an atomic ratio of hydrogen to carbon is 1.51 and the mean calorific value of kukersite is 3600 kcal/kg.
The principal organic component of kukersite is telalginite, derived from the fossil green alga, Gloeocapsomorpha prisca, which has affinities with the modern cyanobacterium, Entophysalis major, an extant species that forms algal mats in inter-tidal to very shallow subtidal waters. Matrix minerals dominantly include low-magnesium calcite, dolomite, and siliciclastic minerals. They are not rich in heavy metals. Kukersite was deposited in a shallow marine basin. It lays in the depth of 7 to 170 metres (23 to 558 ft).
What is Torbanite[]
Formation[]
Torbanite, also known as boghead coal, is a variety of fine-grained black oil shale. It usually occurs as lenticular masses, often associated with deposits of Permian coals. Torbanite is classified as lacustrine type oil shale.
Organic matter (telalginite) in torbanite is derived from lipid-rich microscopic plant remains similar in appearance to the fresh-water colonial green alga Botryococcus braunii. This evidence and extracellular hydrocarbons produced by the alga have led scientists to examine the alga as a source of Permian torbanites and a possible producer of biofuels. Torbanite consists of subordinate amounts of vitrinite and inertinite; however, their occurrence vary depending of deposits.
Torbanite typically comprises 88% carbon and 11% hydrogen. Paraffin oil can be distilled from some forms of torbanite, a process discovered and patented by James Young in 1851.
Location[]
Torbanite is named after Torbane Hill near Bathgate in Scotland, its main location of occurrence. Other major deposits of torbanite are found in Pennsylvania and Illinois, USA, in Mpumalanga in South Africa, in the Sydney Basin of New South Wales, Australia, the largest deposit of which is located at Glen Davis, and in Nova Scotia, Canada.
What is natural a tar (AKA- pitch) pit[]

Gas bubble slowly emerging at La Brea Tar Pits.
A tar pit, pitch pit, or more accurately an asphalt pit or asphalt lake, is the result of a type of petroleum seep where subterranean bitumen leaks to the surface, creating a large area of natural asphalt. This happens because, after the material reaches the surface, its lighter components vaporize, leaving only the thick asphalt.
Paleontological significance[]
Animals usually cannot escape from the asphalt when they fall in, making these pits excellent places to excavate bones of prehistoric animals. The tar pits can trap animals because the asphalt that seeps up from underground forms a bitumen pit so thick that even mammoths could not free themselves before they died of starvation, exhaustion from trying to escape, or exposure to the sun's heat. Over a million fossils have been found in tar pits around the globe.
For other rich deposits, fossilized where they occurred, see Lagerstätten.
Living organisms[]
Living bacteria have been found in the La Brea Tar Pits. These organisms have been shown to be strains of previously discovered bacteria. They have been able to survive and thrive in an environment with no water and little to no oxygen. Scientists started looking for the bacteria when they noticed bubbles of methane coming out of the tar pits.
Other microorganisms have been found living in microliter-sized droplets of water recovered from Pitch Lake in Trinidad, including bacteria from the orders Burkholderiales and Enterobacteriales.
Helaeomyia petrolei, the petroleum fly, spends its larval stage within the tar pit.
Location and formation[]
The La Brea Tar Pits are a group of tar pits around which Hancock Park was formed in urban Los Angeles. Natural asphalt (also called asphaltum, bitumen, pitch or tar—brea in Spanish) has seeped up from the ground in this area for tens of thousands of years. The tar is often covered with dust, leaves, or water. Over many centuries, the bones of animals that were trapped in the tar were preserved. The George C. Page Museum is dedicated to researching the tar pits and displaying specimens from the animals that died there. The La Brea Tar Pits is a registered National Natural Landmark.
The La Brea Tar Pits and Hancock Park are situated within what was once the Mexican land grant of Rancho La Brea, now part of urban Los Angeles in the Miracle Mile district, adjacent to the Los Angeles County Museum of Art and the Craft and Folk Art Museum.
The tar pits visible today are actually from human excavation. The lake pit was originally an asphalt mine. The other pits visible today were produced between 1913 and 1915, when over 100 pits were excavated in search of large mammal bones. Various combinations of asphaltum and water have since filled in these holes. Normally, the asphalt appears in vents, hardening as it oozes out, to form stubby mounds. These can be seen in several areas of the park.
Tar pits are composed of heavy oil fractions called gilsonite, which seeped from the Earth as oil. In Hancock Park, crude oil seeps up along the 6th Street Fault from the Salt Lake Oil Field, which underlies much of the Fairfax District north of the park. The oil reaches the surface and forms pools at several locations in the park, becoming asphalt as the lighter fractions of the petroleum biodegrade or evaporate.
This seepage has been happening for tens of thousands of years. From time to time, the asphalt would form a deposit thick enough to trap animals, and the surface would be covered with layers of water, dust, or leaves. Animals would wander in, become trapped, and eventually die. Predators would enter to eat the trapped animals and also become stuck.
As the bones of dead animals sink into the asphalt, it soaks into them, turning them a dark-brown or black color. Lighter fractions of petroleum evaporate from the asphalt, leaving a more solid substance, which encases the bones. Dramatic fossils of large mammals have been extricated from the tar, but the asphalt also preserves microfossils: wood and plant remnants, rodent bones, insects, mollusks, dust, seeds, leaves, and even pollen grains. Examples of some of these are on display in the George C. Page museum.
Radiometric dating of preserved wood and bones has given an age of 38,000 years for the oldest known material from the La Brea seeps. The pits still ensnare organisms today, so most of the pits are fenced to protect humans and animals.
Formerly at the Biblical 'Lake Asphaltitis' near the Dead Sea. It was also used as a alternate for the Dead Sea. The name for both was fist coined by Titus Flavius Josephus.
What is natural asphalt/bitumen[]

Natural asphalt/bitumen from the Dead Sea.

refined asphalt/bitumen.

The University of Queensland pitch drop experiment, demonstrating the viscosity of asphalt/bitumen.

The road surface is removed and a new bitumen layer is added using a cold milling machine (W 2000). Author: ProjectManhattan.
Note: The terms bitumen and asphalt are mostly interchangeable, except where asphalt is used as an abbreviation for asphalt concrete/tarmac. This article uses "asphalt/bitumen" where either term is acceptable.
Asphalt (US Listeni/ˈæsfɔːlt/, UK /ˈæsfælt/, occasionally /ˈæʃfɔːlt/), also known as bitumen (US /bɪˈtjuːmən, baɪ-/, UK /ˈbɪtjᵿmən/) is a sticky, black and highly viscous liquid or semi-solid form of petroleum. It may be found in natural deposits or may be a refined product; it is a substance classed as a pitch. Until the 20th century, the term asphaltum was also used. The word is derived from the Ancient Greek ἄσφαλτος ásphaltos.
The primary use (70%) of asphalt/bitumen is in road construction, where it is used as the glue or binder mixed with aggregate particles to create asphalt concrete. Its other main uses are for bituminous waterproofing products, including production of roofing felt and for sealing flat roofs.
The terms asphalt and bitumen are often used interchangeably to mean both natural and manufactured forms of the substance. In American English, asphalt (or asphalt cement) is the carefully refined residue from the distillation process of selected crude oils. Outside the United States, the product is often called bitumen. Geologists often prefer the term bitumen. Common usage often refers to various forms of asphalt/bitumen as "tar", such as at the La Brea Tar Pits. Another archaic term for asphalt/bitumen is "pitch".
Naturally occurring asphalt/bitumen is sometimes specified by the term "crude bitumen". Its viscosity is similar to that of cold molasses while the material obtained from the fractional distillation of crude oil boiling at 525 °C (977 °F) is sometimes referred to as "refined bitumen". The Canadian province of Alberta has most of the world's reserves of natural bitumen, covering 142,000 square kilometres (55,000 sq mi), an area larger than England.
Compostition[]
The components of asphalt are classified into four classes of compounds:
- Saturates, saturated hydrocarbons, the % saturates correlates with softening point of the material
- Naphthene aromatics, consisting of partially hydrogenated polycyclic aromatic compounds.
- Polar aromatics, consisting of high molecular weight phenols and carboxylic acids
- Asphaltenes, consisting of high molecular weight phenols and heterocyclic compounds
The naphthene aromatics and polar aromatics are typically the majority components. Additionally, most natural bitumens contain organosulfur compounds, resulting in an overall sulfur content of up to 4%. Nickel and vanadium are found in the <10 ppm level, as is typical of some petroleum.
The substance is soluble in carbon disulfide. It is commonly modelled as a colloid, with asphaltenes as the dispersed phase and maltenes as the continuous phase. and "it is almost impossible to separate and identify all the different molecules of asphalt, because the number of molecules with different chemical structure is extremely large".
Asphalt/bitumen can sometimes be confused with "coal tar", which is a visually similar black, thermoplastic material produced by the destructive distillation of coal. During the early and mid-20th century when town gas was produced, coal tar was a readily available byproduct and extensively used as the binder for road aggregates. The addition of tar to macadam roads led to the word tarmac, which is now used in common parlance to refer to road-making materials. However, since the 1970s, when natural gas succeeded town gas, asphalt/bitumen has completely overtaken the use of coal tar in these applications. Other examples of this confusion include the La Brea Tar Pits and the Canadian oil sands, both of which actually contain natural bitumen rather than tar. Pitch is another term sometimes used at times to refer to asphalt/bitumen, as in Pitch Lake.
Formation and occurrence[]
The great majority of asphalt used commercially is obtained from petroleum. Nonetheless, large amounts of asphalt occur in concentrated form in nature. Naturally occurring deposits of asphalt/bitumen are formed from the remains of ancient, microscopic algae (diatoms) and other once-living things. These remains were deposited in the mud on the bottom of the ocean or lake where the organisms lived. Under the heat (above 50 °C) and pressure of burial deep in the earth, the remains were transformed into materials such as asphalt/bitumen, kerogen, or petroleum.
Natural deposits of asphalt/bitumen include lakes such as the Pitch Lake in Trinidad and Tobago and Lake Bermudez in Venezuela. Natural seeps of asphalt/bitumen occur in the La Brea Tar Pits and in the Dead Sea.
Asphalt/bitumen also occurs in unconsolidated sandstones known as "oil sands" in Alberta, Canada, and the similar "tar sands" in Utah, US. The Canadian province of Alberta has most of the world's reserves of natural bitumen, in three huge deposits covering 142,000 square kilometres (55,000 sq mi), an area larger than England or New York state. These bituminous sands contain 166 billion barrels (26.4×109 m3) of commercially established oil reserves, giving Canada the third largest oil reserves in the world. and produce over 2.3 million barrels per day (370×103 m3/d) of heavy crude oil and synthetic crude oil. Although historically it was used without refining to pave roads, nearly all of the bitumen is now used as raw material for oil refineries in Canada and the United States.
The world's largest deposit of natural bitumen, known as the Athabasca oil sands is located in the McMurray Formation of Northern Alberta. This formation is from the early Cretaceous, and is composed of numerous lenses of oil-bearing sand with up to 20% oil. Isotopic studies attribute the oil deposits to be about 110 million years old. Two smaller but still very large formations occur in the Peace River oil sands and the Cold Lake oil sands, to the west and southeast of the Athabasca oil sands, respectively. Of the Alberta bitumen deposits, only parts of the Athabasca oil sands are shallow enough to be suitable for surface mining. The other 80% has to be produced by oil wells using enhanced oil recovery techniques like steam-assisted gravity drainage.
Much smaller heavy oil or bitumen deposits also occur in the Uinta Basin in Utah, US. The Tar Sand Triangle deposit, for example, is roughly 6% bitumen.
Asphalt/bitumen occurs in hydrothermal veins. An example of this is within the Uinta Basin of Utah, in the US, where there is a swarm of laterally and vertically extensive veins composed of a solid hydrocarbon termed Gilsonite. These veins formed by the polymerization and solidification of hydrocarbons that were mobilized from the deeper oil shales of the Green River Formation during burial and diagenesis.
Asphalt/bitumen is similar to the organic matter in carbonaceous meteorites. However, detailed studies have shown these materials to be distinct. The vast Alberta bitumen resources are believed to have started out as living material from marine plants and animals, mainly algae, that died millions of years ago when an ancient ocean covered Alberta. They were covered by mud, buried deeply over the eons, and gently cooked into oil by geothermal heat at a temperature of 50 to 150 °C (120 to 300 °F). Due to pressure from the rising of the Rocky Mountains in southwestern Alberta, 80 to 55 million years ago, the oil was driven northeast hundreds of kilometres into underground sand deposits left behind by ancient river beds and ocean beaches, thus forming the oil sands.
What is 'tight oil'[]
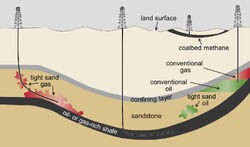
Shown are conceptual illustrations of types of oil and gas wells. A vertical well is producing from a conventional oil and gas deposit (right). Also shown are wells producing from unconventional formations: a vertical coalbed methane well (second from right); a horizontal well producing from a shale formation (center); and a well producing from a tight sand formation (left).
Formation[]
Tight oil (also known as shale oil or light tight oil, abbreviated LTO) is petroleum that consists of light crude oil contained in petroleum-bearing formations of low permeability, often shale or tight sandstone. Economic production from tight oil formations requires the same hydraulic fracturing and often uses the same horizontal well technology used in the production of shale gas. It should not be confused with oil shale, which is shale rich in kerogen, or shale oil, which is oil produced from oil shales.
Therefore, the International Energy Agency recommends to use the term "light tight oil" for oil produced from shales or other very low permeability formations, while World Energy Resources 2013 report by the World Energy Council uses the term "tight oil".
Economics[]
In May 2013 the International Energy Agency in its Medium-Term Oil Market Report (MTOMR) said that the North American oil production surge led by unconventional oils - US light tight oil (LTO) and Canadian oil sands - had produced a global supply shock that would reshape the way oil is transported, stored, refined and marketed.
Location[]
Following are estimates of technically recoverable volumes of tight oil associated with shale formations, made by the US Energy Information Administration in 2013. Not all oil which is technically recoverable may be economically recoverable at current or anticipated prices.
- Russia: 75 billion barrels
- United States: 48 to 58 billion barrels
- China: 32 billion barrels
- Argentina: 27 billion barrels
- Libya: 26 billion barrels
- Venezuela: 13 billion barrels
- Mexico: 13 billion barrels
- Pakistan: 9 billion barrels
- Canada: 9 billion barrels
- Indonesia: 8 billion barrels
- World Total 335 to 345 billion barrels
- A Australian private oil company announced that it had discovered tight oil in shale of the Arckaringa Basin, which they estimated at 3.5 to 223 billion barrels.
What is heavy crude oil (or extra heavy crude oil)[]
Heavy crude oil (or extra heavy crude oil) is highly-viscous oil that cannot easily flow to production wells under normal reservoir conditions.(Mai)
It is referred to as "heavy" because its density or specific gravity is higher than that of light crude oil. Heavy crude oil has been defined as any liquid petroleum with an API gravity less than 20°.(Dusseault 2001) Physical properties that differ between heavy crude oils and lighter grades include higher viscosity and specific gravity, as well as heavier molecular composition. In 2010, the World Energy Council defined extra heavy oil as crude oil having a gravity of less than 10° and a reservoir viscosity of no more than 10,000 centipoises. When reservoir viscosity measurements are not available, extra-heavy oil is considered by the WEC to have a lower limit of 4° °API.(WEC 2007) (i.e. with density greater than 1000 kg/m3 or, equivalently, a specific gravity greater than 1 and a reservoir viscosity of no more than 10,000 centipoises. Heavy oils and asphalt are dense nonaqueous phase liquids (DNAPLs). They have a "low solubility and are with viscosity lower and density higher than water.(2003 & Llamas 118) "Large spills of DNAPL will quickly penetrate the full depth of the aquifer and accumulate on its bottom."(2008 & Vrba 23)
Related substances[]
Heavy crude oil is closely related to natural bitumen from oil sands. Petroleum geologists categorize bitumen from oil sands as ‘extra-heavy oil’ due to its density of less than 10° °API. Bitumen is the heaviest, thickest form of petroleum. According to the U.S. Geological Survey, bitumen is further distinguished as extra-heavy oil with a higher viscosity (i.e., resistance to flow): “Natural bitumen, also called tar sands or oil sands, shares the attributes of heavy oil but is yet more dense and viscous. Natural bitumen is oil having a viscosity greater than 10,000 cP.” “Natural bitumen (often called tar sands or oil sands) and heavy oil differ from light oils by their high viscosity (resistance to flow) at reservoir temperatures, high density (low API gravity), and significant contents of nitrogen, oxygen, and sulfur compounds and heavy-metal contaminants. They resemble the residuum from the refining of light oil. Most heavy oil is found at the margins of geologic basins and is thought to be the residue of formerly light oil that has lost its light-molecular-weight components through degradation by bacteria, water-washing, and evaporation. Conventional heavy oil and bitumens differ in the degree by which they have been degraded from the original crude oil by bacteria and erosion.(Meyer & 2003 1) Often, bitumen is more viscous than cold molasses and does not flow at ambient conditions.
According to World Resources Institute, concentrations of remarkable quantities of heavy oil and oil sands are found in Canada and Venezuela. The U.S. Energy Information Administration (EIA) reported in 2001 that the largest reserves of heavy crude oil in the world were located north of the Orinoco river 270-mile long by 40-mile wide Orinoco Belt in eastern Venezuela. At that time Venezuela began authorizing "joint ventures to upgrade the extra-heavy crude resources." Petroleos de Venezuela, S.A. (PDVSA) at that time estimated that there were 270 billion barrels of recoverable reserves in the area, the same amount as the conventional oil reserves of Saudi Arabia. The Orinoco Belt in Venezuela is sometimes described as oil sands, but these deposits are non-bituminous, falling instead into the category of heavy or extra-heavy oil due to their lower viscosity. Natural bitumen and extra-heavy oil differ in the degree by which they have been degraded from the original conventional oils by bacteria. According to the WEC, extra-heavy oil has "a gravity of less than 10° °API and a reservoir viscosity of no more than 10,000 centipoise". Thirty or more countries are known to have reserves.
Production, transportation, and refining of heavy crude oil present special challenges compared to light crude oil. Generally, a diluent is added at regular distances in a pipeline carrying heavy crude to facilitate its flow. Dilbit (diluted bitumen) is a means of transporting highly viscous hydrocarbon. Per the Alberta Oil Sands Bitumen Valuation Methodology, "Dilbit Blends" means "Blends made from heavy crudes and/or bitumens and a diluent usually condensate, for the purpose of meeting pipeline viscosity and density specifications, where the density of the diluent included in the blend is less than 800 kg/m3."
Chemical properties[]
Heavy oil is asphaltic and contains asphaltenes and resins. It is "heavy" (dense and viscous) due to the high ratio of aromatics and naphthenes to linear alkanes and high amounts of NSO's (nitrogen, sulfur, oxygen and heavy metals). Heavy oil has a higher percentage of compounds with over 60 carbon atoms and hence a high boiling point and molecular weight. For example, the viscosity of Venezuela's Orinoco extra-heavy crude oil lies in the range 1000–5000 cP (1–5 Pa·s), while Canadian extra-heavy crude has a viscosity in the range 5000–10,000 cP (5–10 Pa·s), about the same as molasses, and higher (up to 100,000 cP or 100 Pa·s for the most viscous commercially exploitable deposits). A definition from the Chevron Phillips Chemical company is as follows:
The "heaviness" of heavy oil is primarily the result of a relatively high proportion of a mixed bag of complex, high molecular weight, non-paraffinic compounds and a low proportion of volatile, low molecular weight compounds. Heavy oils typically contain very little paraffin and may or may not contain high levels of asphaltenes.
Heavy crude oil is generally categorized in two ways:
- Those that have over 1% sulfur (high sulfur crude oils), with aromatics and asphaltenes, and these are mostly found in North America (Canada (Alberta, Saskatchewan), United States (California), Mexico), South America (Venezuela, Colombia and Ecuador) and the Middle East (Kuwait, Saudi Arabia).
- Those that have less than 1% sulfur (low sulfur crude oils), with aromatics, naphthenes and resins, and these are mostly found in Western Africa (Chad), Central Africa (Angola) and East Africa (Madagascar).
Geological origin[]
Most geologists agree that crude becomes ‘heavy’ as a result of biodegradation, in which lighter ends are preferentially consumed by bacterial activity in the reservoir, leaving heavier hydrocarbons behind. This hypothesis leans heavily on the techniques of petroleum geochemistry. Poor geologic reservoir sealingexposes the hydrocarbon to surface contaminants, including organic life (such as bacteria) and contributes to this process.
Heavy oils can be found in shallow, young reservoirs, with rocks from the Pleistocene, Pliocene, and Miocene (younger than 25 million years). In some cases, it can also be found in older Cretaceous, Mississippian, and Devonian reservoirs. These reservoirs tend to be poorly sealed, resulting in heavy oil and oil-sands.
Economics[]

Refinery using the Shukhov cracking process, Baku, Soviet Union, 1934.
Heavy crude oils provide an interesting situation for the economics of petroleum development. The resources of heavy oil in the world are more than twice those of conventional light crude oil. In October 2009, the United States Geological Survey updated the Orinoco deposits (Venezuela) recoverable value to 513 billion barrels (8.16×1010 m3), making this area one of the world's largest recoverable oil deposits. However, recovery rates for heavy oil are often limited from 5-30% of oil in place. The chemical makeup is often the defining variable in recovery rates. The technology utilized for the recovery of heavy oil has steadily increased recovery rates. The oil can be chemicaly 'cracked' to produce other 'lighter' substances like petrol and LPG.
In petroleum geology and chemistry, cracking is the process whereby complex organic molecules such as kerogens or long chain hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of carbon-carbon bonds in the precursors. The rate of cracking and the end products are strongly dependent on the temperature and presence of catalysts. Cracking is the breakdown of a large alkane into smaller, more useful alkanes and alkenes. Simply put, hydrocarbon cracking is the process of breaking a long-chain of hydrocarbons into short ones. This process might require high temperatures and high pressure.
More loosely, outside the field of petroleum chemistry, the term "cracking" is used to describe any type of splitting of molecules under the influence of heat, catalysts and solvents, such as in processes of destructive distillation or pyrolysis.
Fluid catalytic cracking produces a high yield of petrol and LPG, while hydrocracking is a major source of jet fuel, Diesel fuel, naphtha, and again yields LPG.
On one hand, due to increased refining costs and high sulfur content for some sources, heavy crudes are often priced at a discount to lighter ones. The increased viscosity and density also makes production more difficult (see reservoir engineering). On the other hand, large quantities of heavy crudes have been discovered in the Americas, including Canada, Venezuela and California. The relatively shallow depth of heavy oil fields (often less than 3000 feet) can contribute to lower production costs; however, these are offset by the difficulties of production and transport that render conventional production methods ineffective. Specialized techniques are being developed for exploration and production of heavy oil.
There usage[]
Historic examples[]
Humans have used oil shale as a fuel since prehistoric times, since it generally burns without any processing. Ancient Britons of the Iron Age also used to polish it and form it into ornaments.
In England a member of the Bradshaigh family discovered a plentiful shallow seam of smooth, hard, cannel coal on his estate, in Haigh, Greater Manchester in the 16th century. The shallow depth at which it was found meant it was suitable for the simple surface mining methods available at that time. It could be worked and carved, and was prized for fireplaces as an excellent fuel that burned with a bright flame, was easily lit, and left virtually no ash.
The first patent for extracting oil from oil shale was British Crown Patent 330 granted in 1694 to three persons named Martin Eele, Thomas Hancock and William Portlock who had:
- "found a way to extract and make great quantities of pitch, tarr, and oyle out of a sort of stone."
In Canada, the First Nation peoples had used bitumen from seeps along the Athabasca and Clearwater Rivers to waterproof their birch bark canoes from early prehistoric times. The Canadian oil sands first became known to Europeans in 1719 when a Cree native named Wa-Pa-Su brought a sample to Hudsons Bay Company fur trader Henry Kelsey, who commented on it in his journals. Fur trader Peter Pond paddled down the Clearwater River to Athabasca in 1778, saw the deposits and wrote of "springs of bitumen that flow along the ground." In 1787, fur trader and explorer Alexander MacKenzie on his way to the Arctic Ocean saw the Athabasca oil sands, and commented, "At about 24 miles from the fork (of the Athabasca and Clearwater Rivers) are some bituminous fountains into which a pole of 20 feet long may be inserted without the least resistance."
The Athabasca River cuts through the heart of the deposit, and traces of the heavy oil are readily observed as black stains on the river banks. Since portions of the Athabasca sands are shallow enough to be surface-mineable, they were the earliest ones to see development. Historically, the bitumen was used by the indigenous Cree and Dene Aboriginal peoples to waterproof their canoes. The Athabasca oil sands first came to the attention of European fur traders in 1719 when Wa-pa-su, a Cree trader, brought a sample of bituminous sands to the Hudson's Bay Company post at York Factory on Hudson Bay.
Modern industrial mining of oil shale began in 1837 in Autun, France, followed by exploitation in Scotland, Germany, America and several other countries. The oil shale called Torbanite typically comprises 88% carbon and 11% hydrogen. Paraffin oil can be distilled from some forms of torbanite, a process discovered and patented by James Young in 1851.
Operations during the 19th century focused on the production of kerosene, lamp oil, and paraffin; these products helped supply the growing demand for lighting that arose during the Industrial Revolution. Fuel oil, lubricating oil and grease, and ammonium sulfate were also produced. The European oil-shale industry expanded immediately before World War I due to limited access to conventional petroleum resources and to the mass production of automobiles and trucks, which accompanied an increase in gasoline consumption.
WW1 and WW2 usage[]

Autun oil shale mines.
Although the Estonian and Chinese oil-shale industries continued to grow after World War II, most other countries abandoned their projects due to high processing costs and the availability of cheaper petroleum.
China had discovered the outer edges of the region's oil, oil shale, oil sand, coal and natural gas reserves in 1929. Oil shale were extracted in rising amounts between 1929 to 1937, when it leveled off until 1944, with a fall in 1945. Tarakan crude oil from Bornio was mixed with lesser quantities of Manchurian oil shale distillates to make the primary feedstock for the production of Japanese diesel fuel in 1942. Australian troops ended the occupation of Tarakan and surrounding parts of Borneo in the June of 1945.
Romania had been a major power in the oil industry since the 1800s. It was one of the largest producers in Europe and Ploiesti was a major part of that production. (see Bombing of Romania in World War II). The Ploiești oil refineries provided about 30% of all Axis oil production.
Operation Tidal Wave was an air attack by bombers of the United States Army Air Forces (USAAF) based in Libya and Southern Italy on nine oil refineries around Ploiești, Romania on 1 August 1943, during World War II. It was a strategic bombing mission and part of the Allies' "oil campaign" to deny petroleum-based fuel to the Axis. The mission resulted in "no curtailment of overall product output".
Romania discovered some oil sands and shale near the oil producing town of Ploiești in the run up to WW2 and plans were made to use them if the conventional oil ran out, but it did not.
Cold War usage[]
The then high oil prices, hostile Arabs and falling supplies in the 1970s lead to a plan to exploit many unusual, remote and/or marginal fields in order to secure oil new supplies and as a possible way of earning more revenue as rising prices made them economically viable.
Following the 1973 oil crisis, world production of oil shale reached a peak of 46 million tonnes in 1980 before falling to about 16 million tonnes in 2000, due to competition from cheap conventional petroleum in the 1980s.
It would also have provided a theoretical alternative if the Middle East went crazy and the Gulf States were destroyed.
On 2 May 1982, known in some circles as "Black Sunday", Exxon canceled its US$5 billion Colony Shale Oil Project near Parachute, Colorado because of low oil-prices and increased expenses, laying off more than 2,000 workers and leaving a trail of home-foreclosures and small-business bankruptcies. In 1986, President Ronald Reagan signed into law the Consolidated Omnibus Budget Reconciliation Act of 1985 which among other things abolished the United States' Synthetic Liquid Fuels Program.
Modern usage[]
The global oil-shale industry began to revive at the beginning of the 21st century. In 2003, an oil-shale development program restarted in the United States. Authorities introduced a commercial leasing program permitting the extraction of oil shale and oil sands on federal lands in 2005, in accordance with the Energy Policy Act of 2005.
Venezuela's Orinoco Belt was discovered in 2003 and has been experimentally tapped for oil and gas since 2003. Oil sand/shale mining was planned for 2013, but is now planned for some time in the the 2020s.
As of 2008, industry uses oil shale in Brazil, China, Estonia and to some extent in Germany, and Russia. Several additional countries started assessing their reserves or had built experimental production plants, while others had phased out their oil shale industry. Oil shale serves for oil production in Estonia, Brazil, and China; for power generation in Estonia, China, and Germany; for cement production in Estonia, Germany, and China; and for use in chemical industries in China, Estonia, and Russia.As of 2009, 80% of oil shale used globally is extracted in Estonia, mainly due to the Oil-shale-fired power plants.
Oil shale serves as the main fuel for power generation only in Estonia, where the oil-shale-fired Narva Power Plants accounted for 95% of country's electrical generation in 2005.
Individual fields, reserves, beds and basins[]
Athabasca oil sands and shales[]
The Athabasca oil sands in Alberta, Canada, are a very large source of bitumen, which can be chemically upgraded in a upgrader unit to synthetic crude oil.
- Country- Canada
- Region- Northern Alberta
- Offshore/onshore- Onshore, mining
- Coordinates- 57.02°N 111.65°WCoordinates: 57.02°N 111.65°W
- Site operators- Syncrude, Suncor, CNRL, Shell, Total, Imperial Oil, Petro Canada, Devon, Husky, Statoil and Nexen
- Corporate partners- Chevron, Marathon, ConocoPhillips, BP and Oxy
- Discovery- 1848
- Start of production- 1967
- Current production of oil- 1,300,000 barrels per day (~6.5×107 t/a)
- Estimated oil in place- 133,000 million barrels (~1.81×1010 t)
- Producing formations- McMurray, Clearwater and Grand Rapids
The Athabasca oil sands are large deposits of bitumen or extremely heavy crude oil, located in northeastern Alberta, Canada – roughly centred on the boomtown of Fort McMurray. These oil sands, hosted primarily in the McMurray Formation, consist of a mixture of crude bitumen (a semi-solid rock-like form of crude oil), silica sand, clay minerals, and water. The Athabasca deposit is the largest known reservoir of crude bitumen in the world and the largest of three major oil sands deposits in Alberta, along with the nearby Peace River and Cold Lake deposits (the latter stretching into Saskatchewan).
Together, these oil sand deposits lie under 141,000 square kilometres (54,000 sq mi) of boreal forest and muskeg (peat bogs) and contain about 1.7 trillion barrels (270×109 m3) of bitumen in-place, comparable in magnitude to the world's total proven reserves of conventional petroleum. The International Energy Agency (IEA) lists the economically recoverable reserves, at 2006 prices and modern unconventional oil production technology, to be 178 billion barrels (28.3×109 m3), or about 10% of these deposits. These contribute to Canada's total proven reserves being the third largest in the world, after Saudi Arabia and Venezuela's Orinoco Belt.
In Canada, the First Nation peoples had used bitumen from seeps along the Athabasca and Clearwater Rivers to waterproof their birch bark canoes from early prehistoric times. The Canadian oil sands first became known to Europeans in 1719 when a Cree native named Wa-Pa-Su brought a sample to Hudsons Bay Company fur trader Henry Kelsey, who commented on it in his journals. Fur trader Peter Pond paddled down the Clearwater River to Athabasca in 1778, saw the deposits and wrote of "springs of bitumen that flow along the ground." In 1787, fur trader and explorer Alexander MacKenzie on his way to the Arctic Ocean saw the Athabasca oil sands, and commented, "At about 24 miles from the fork (of the Athabasca and Clearwater Rivers) are some bituminous fountains into which a pole of 20 feet long may be inserted without the least resistance."
The Athabasca River cuts through the heart of the deposit, and traces of the heavy oil are readily observed as black stains on the river banks. Since portions of the Athabasca sands are shallow enough to be surface-mineable, they were the earliest ones to see development. Historically, the bitumen was used by the indigenous Cree and Dene Aboriginal peoples to waterproof their canoes. The Athabasca oil sands first came to the attention of European fur traders in 1719 when Wa-pa-su, a Cree trader, brought a sample of bituminous sands to the Hudson's Bay Company post at York Factory on Hudson Bay.
Minor exploration and mining activity started up for a while in 1900. Operations were mooted in 1924-26, 1940, 1958 and 1962. The 1962 plans were take up in 1965 and enacted in 1967. It's development was inhibited by the declining world oil prices in the late 1960s. The second mine, operated by the Syncrude consortium, did not begin operating until 1978, after the 1973 oil crisis had caused prices to rise, thus sparking investors' interest.
By 2009, the two extraction methods used were in situ extraction, when the bitumen occurs deeper within the ground, (which will account for 80 percent of oil sands development) and surface or open-pit mining, when the bitumen is closer to the surface. Only 20 percent of bitumen can be extracted using open pit mining methods, which involves large scale excavation of the land with huge hydraulic power shovels and 400-ton heavy hauler trucks. Surface mining leaves toxic tailings ponds. In contrast, in situ uses more specialized techniques such as steam-assisted gravity drainage (SAGD). "Eighty percent of the oil sands will be developed in situ which accounts for 97.5 percent of the total surface area of the oil sands region in Alberta." In 2006 the Athabasca deposit was the only large oil sands reservoir in the world which was suitable for large-scale surface mining, although most of this reservoir can only be produced using more recently developed in-situ technology.
Critics contend that government and industry measures taken to reduce environmental and health risks posed by large-scale mining operations are inadequate, causing unacceptable damage to the natural environment and human welfare. Objective discussion of the environmental impacts has often been clouded by polarised arguments from industry and from advocacy groups.
Melville Island oil shales[]
A map of Melville Island, Northwest Territories/Nunavut, Canada.
Melville Island (Northwest Territories and Nunavut) is a uninhabited island of the Canadian Arctic Archipelago with an area of 42,149 km2 (16,274 sq mi). It is the 33rd largest island in the world and Canada's eighth largest island. Melville Island is shared by the Northwest Territories, which is responsible for the western half of the island, and Nunavut, which is responsible for the eastern half. The border runs along the 110th meridian west. The mountains on Melville Island, some of the largest in the western Canadian Arctic, reach heights of 1,000 m (3,300 ft). There are two subnational pene-exclaves that lie west of the 110th meridian and form part of the Northwest Territories. These can only be reached by land from Nunavut or boat from the Northwest Territories.
Melville has surfaced as a candidate for natural gas deposits. The island was believed to have deposits of coal and oil shale since the first half of the 20th century. The first Canadian Arctic island exploratory well was spudded in 1961 at Winter Harbour. It drilled Lower Paleozoic strata to a total depth of 3,823 m (12,543 ft). In the 1970s, the northern portion of the island on the east side of the Sabine Peninsula proved to contain a major gas field, known as Drake Point. The lease was owned by Panarctic Oils, a joint operation with the Canadian Government.
Utah oil sands[]
The Utah Tar Sands.

Mountains of the Uintah Basin in Utah.
In the United States a large supply of oil sands are found in Eastern Utah. These deposits of bitumenor heavy crude oil have the ability to generate about 12 to 19 billion barrels from a number of prominent sites. the Oil Shale and Tar Sands Programmatic EIS Information Center had confirmed most of this by 2008.
Since the early 1900s the oil sand deposits have been extracted mainly for the use of road pavement. Later, in the 1970s, oil companies began to experiment with the deposits in the hope of using it for their benefit. These experiments ended in the late 1980s when the technologies being used were concluded inefficient and too expensive. Recently, oil companies have again become interested in Utah's oil sands. Now that conventional oil is becoming harder to find, oil sands have become an alternative fuel source.
Utah's oil sands are made up of several different deposits all consisting of different amounts of heavy or crude oil. These sites are mostly found on public lands. They are mainly close together and many are found within the Uintah Basin of Utah, which is a section of the Colorado Plateaus province. Some of these sites include Sunnyside, P.R. Spring, Asphalt Ridge, Hill Creek, Circle Ridge, Circle Cliffs, White Rocks, and the Tar Sand Triangle, the highest deposit.
The Tar Sand Triangle is located in Southeastern Utah and covers an area of 148,000 acres. It is located between the Dirty Devil and Colorado Rivers in Wayne and Garfield Counties. The Tar Sand Triangle is the largest deposit of oil sands in the United States known today. It contains about 6.3 billion barrels of heavy oil, but is thought to have originally held more. At one point the Tar Sand Triangle could have consisted of 16 billion barrels of heavy oil, almost as much as in Utah today.
In the United States a large supply of oil sands are found in Eastern Utah. These deposits of bitumen or heavy crude oil have the ability to generate about 12 to 19 billion barrels from a number of prominent sites.
Since the early 1900s the oil sand deposits have been extracted mainly for the use of road pavement. Later, in the 1970s, oil companies began to experiment with the deposits in the hope of using it for their benefit. These experiments ended in the late 1980s when the technologies being used were concluded inefficient and too expensive. Recently, oil companies have again become interested in Utah's oil sands. Now that conventional oil is becoming harder to find, oil sands have become an alternative fuel source.
The Utah Oil Sands Joint Venture is a joint venture between Nevtah Capital Management, Inc., and Black Sands Energy Corp. to develop oil sands resources at the Uintah Basin in Utah.
Oil-sands extraction in Utah started in the 1960s when two extraction plants were constructed. Western Industries opened a strip-mine and built a pilot plant along the east side of the Whiterocks River and Major Oil Company opened a strip-mine and built a pilot plant on the west side off the Whiterocks River. In 2005, Nevtah Capital Management and Cassandra Energy (now: Black Sands Energy) formed a joint venture to develop Utah's oil sands and opened a pilot plant at the Asphalt Ridge lease location. The pilot plant became in operation in November 2005.
The joint venture uses closed-loop solvent extraction process originally proven by X-TRAC Energy in Wyoming in 1998, with a full-scale production plant. Black Sands Energy has exclusive rights to a technology.
The above-ground extraction process dissolute crushed, 1" minus oil sands materials through contact with a benign non-toxic solvent in an enclosed extractor vessel at temperatures up to 300 °F (149 °C) at near-atmospheric pressures. As the material dissolves, it is passed to a wash chamber where any remaining oil is removed. The oil-free sand is then desolventised with heat, which converts the liquid solvent to a gas, leaving dry solids suitable for mine backfill. The solvent-oil mixture is pumped into a critical unit for the removal of asphalt and oil from the solvent through heating and cooling. The recovered solvent is compressed back to a liquid, cooled and re-circulated to the extractor vessel in an endless loop. The system consists of only few moving parts and it operates on a gravity principle. Since the process does not use water to recover the oil, energy requirements are minimal.
The partnership holds the rights to 13 oil sands leases in Utah consisting of 11,535 acres (46.68 km2) containing over 650,000,000 bbl of recoverable oil.
The joint venture owns a 200 bbl per day mobile pilot plant and preparing a 2,000 bbl per day commercial production unit. The production capacity is expected to increase up 50,000 bbl per day by the end of 2009. The system has been improved to maintain processing levels at cold temperatures. A steam jacket has been installed which creates drier sand and keeps the pumps, plumbing and the extraction chamber warmer during standby time, minimizing warm-up time. System performance has improved with the installation of more powerful pumps and additional sensors for better indications of mass flow, temperature and material levels. The upgraded process control provides more precise data required in order to measure the system's performance.
The partnership is between Nevtah Capital Management, Inc., and Black Sands Energy Corp. The extraction technology is provided by development by Black Sands Energy nd the financing is provided by Nevtah Capital Management. On 12 January 2007, Nevtah Capital Management and Black Sands Energy announced a joint venture agreement with Korea Technology Industry. According to the agreement, Korea Technology Industry provides $19 million for the development of the Whiterocks Deposit, in exchange of 50% of net profit. The joint venture agreement is limited to 100 million barrels of oil.
Manchurian oil sands and shales[]
China had discovered the outer edges of the region's oil, oil shale, oil sand, coal and natural gas reserves in 1929. Oil shale were extracted in rising amounts between 1929 to 1937, when it leveled off until 1944, with a fall in 1945. Tarakan crude oil from Bornio was mixed with lesser quantities of Manchurian oil shale distillates to make the primary feedstock for the production of Japanese diesel fuel in 1942. Australian troops ended the occupation of Tarakan and surrounding parts of Borneo in the June of 1945.
China tried to rebuild the Japanese plants and expand on them in 1959-1960 under a plan to increase thier fule supply so as to maintain fule indipendence once industry had eventuly taken of. Plans emerged in the 1970s and early 1980s. Modern activity started with plans in the late 1980 along with the growing industrial sector and exploration started in the 1990's, which in turn lead to the discovery of major finds in the local basin. Test drilling and mining started in the 2000s and major extraction work go underway in the 2010s.
Ploiești oil sands and shales[]
In the mid-19th century the Ploiești region was one of the world's leading oil extraction and refinery sites. The world's first large refinery opened at Ploiești in 1856-1857, with US investment.
Romania had been a major power in the oil industry since the 1800s. It was one of the largest producers in Europe and Ploiești was a major part of that production, hence it's fate in the Allied bombing of Romania in World War II. The Ploiești oil refineries provided about 30% of all Axis oil production.
Romania discovered some oil sands and shale near the oil producing town of Ploiești during the run up to WW2 and plans were made to use them if the conventional oil ran out, but it did not.
Operation Tidal Wave was an air attack by bombers of the United States Army Air Forces (USAAF) based in Libya and Southern Italy on nine oil refineries around Ploiești, Romania on 1 August 1943, during World War II. It was a strategic bombing mission and part of the "oil campaign" to deny petroleum-based fuel to the Axis. The mission resulted in "no curtailment of overall product output".
In 1950, as a milestone in the development of the petroleum, hydrocarbon processing, and petrochemical industries, the Engineering and Design Institute for Oil Refineries and Petrochemical Plants, SC IPIP SA, a Romanian company with a large range of capabilities and experience, was established at Ploiești. The oil and gas field still produces a modest amount of oil to date.
Télots\Autun oil shales[]

The Autun oil shale mines.
The mine of Télots operated the oil shale in Autun which lay on the edge of the city of Autun in Saint-Forgeot, Saône-et-Loire, eastern France.
Extraction begins in 1824 and was produced in 1837 for public lighting and facilities, but it soon diversified production. More mining concessions were granted in 1865.
The refinery was completed in in 1936 and employed several hundred workers who produced fuel for automobiles.
It was sabotaged by the locals and bombed in the ailed Scullion raids. In retaliation the local occupation militia killed five workers.
Upon it's closing due falling reserves in 1957, the site was dismantled and partially demolished. The ruins and two large fly ash\slag heaps mark the landscape still early in the early 21st century, and is invaded by a particular vegetation that is studied for its biodiversity.
Kimmeridge oil shale and clays[]

The "nodding donkey" oil well pump at Kimmeridge Oil Field.
The Kimmeridge Oil Field is to the northwest of Kimmeridge Bay, on the south coast of the Isle of Purbeck, in Dorset, England.
The small village Kimmeridge is about 6 kilometres (3.7 mi) south of Wareham and about 8 kilometres (5.0 mi) west of Swanage. The Kimmeridge oil field is part of the Wytch Farm oil field and processing facility operated by Perenco. Wytch Farm is on the southern shore of Poole Harbour and about 12 kilometres (7.5 mi) northeast of the oil well. Kimmeridge Bay and its cliffs are part of the Jurassic Coast, a World Heritage Site, because of the quality and variety of geological landforms along the coast.
Ancient Britons and Romans used polished 'Black stone', a shiny type of oil shale, for ornaments. Local Romans also burnt the duller stones for fuel in-lieu for coal and wood when supplies were short.
Victorians began mining the shales on a formal basis in around the 1850s for a local source of industrial fule. Oil prospecting began in the 1920s and a reserve was found in 1935. Drilling was at 6 poinis between 1958 and 1980.
In Dorset the search for oil started back in 1935. Between 1958 and 1980 six wells were drilled in Kimmeridge Bay. The first drilling of the Kimmeridge oil field was the Broadbench 1 well to the north of Broadbench, which revealed neither oil nor gas. The second well Broadbench 2, later renamed Kimmeridge 1, was drilled in 1959 and both oil and gas were discovered. The Kimmeridge 2 well (drilled 1960) to the east of the car park showed only small amounts oil, but it was retained as an observation well. The drillings Kimmeridge 3 (drilled 1959 to 1960) at Broad Bench and Kimmeridge 5 (drilled in 1980 near the Kimmeridge 1 well) exposed only weak oil contents, while the drilling Kimmeridge 4 in 1960, east of Brandy Bay by Long Ebb, revealed no exposures due to a mechanical breakdown.
The Kimmeridge 1 well site, is a small site, with a single beam pump or "nodding donkey" which has been pumping continually since 1961, making it the oldest working oil pump in the UK. The well once produced 350 barrels per day, but currently yields around 65 barrels per day (10.3 m3/d) from the Jurassic strata that lie around 350 metres (1,150 ft) below the cliff. The well has been operating this long because it is tapped into a network of connected reserves, however the yield is decreasing. The oil is transported by tanker to Wytch Farm, from where it is piped to the storage tanks at Hamble-le-Rice on Southampton Water before being shipped to the main refinery. The nodding donkey has a high wire-mesh fence around it but it can be viewed clearly from all sides. The well provides views over the Kimmeridge Ledges and 8 kilometres (5.0 mi) of coast where bedrock extends at least half a kilometre out to sea under the waves.
The dried (of water, not oil) clay and shales are still used by locals as a informal domestic fuel.
Isle of Purbeck's oil-shale field[]

Oil wells on the Goathorn Peninsula, Poole Harbour
Wytch Farm is an oil field and processing facility in the Purbeck district of Dorset, England. It is the largest onshore oil field in western Europe. The facility, recently taken over by Perenco was previously operated by BP. It is hidden in a coniferous forest on Wytch Heath on the southern shore of Poole Harbour, two miles (3 km) north of Corfe Castle. Oil and natural gas (methane) are both exported by pipeline; liquefied petroleum gas is exported by road tanker.
The oil field drew its name from the neighbouring Wytch Farm which had existed on the site for many centuries on the fringes of Wytch Heath.
The Isle of Purbeck's oil-shale field, or "Kimmeridge Coal" which has been won from the cliffs to the east of Kimmeridge since the early 17th century, is no longer used commercially. Similar deposits were found at Wytch Farm in the 1890s, but were commercially exploited until only circa 1900, and only at a low level. The Kimmeridge Oil and Carbon Company reported that in 1890 it had dug 5,000 ft of underground tunnels at Kimmeridge on four levels into the local cliffs. There was a local jetty to export the oil shale, and smaller operations occurred at nearby Bencliff Grit east of Osmington Mills.
Isle of Purbeck's oil industry began in 1936 with the first unsuccessful and then experimental wells drilled at Broad Bench near Kimmeridge by D'Arcy Exploration. The area had long been mined for oil shale and tar, but was only prospected for crude oil in the 1950s. It was not until 1959 that a borehole at Kimmeridge showed that oil was seeping out, and 1960 saw British Petroleum's Kimmeridge Oil Field discovered. Oil wells started to be built on the Goathorn Peninsula, Poole Harbour.
The field was discovered by the nationalised British Gas Corporation in December 1973 and began producing oil in 1979. As part of the privatisation of British Gas in the 1980s Wytch Farm was sold to BP which took over as operator in 1984. In May 2011, BP announced that it had agreed to sell its majority interest in Wytch Farm to Perenco, which became the new operator. Premier Oil has a 30.1% stake in the field. BP announced May 17, 2011, the sale of its interests in the Wytch Farm, Wareham, Beacon and Kimmeridge fields to Perenco and the sale of the Dimlington gas terminal to Perenco in February 2011. In September 2012, Perenco UK applied to Dorset County Council (DCC) for permission to extend the life of 39 planning permissions at three of the oilfields. DCC’s Planning Committee recommended approval of the applications on 6 September 2013, thereby extending the operational life of the oilfields beyond their original end-date of 2016 to 2037.
Production grew from 4,000 to 6,000 barrels per day (950 m3/d) (bpd) by 1984[12] and eventually peaked at 110,000 bbl/d (17,000 m3/d) in 1997; by 2002 this had declined to 50,000 bbl/d (7,900 m3/d). In 2002 it was estimated that the field contained reserves of 65.40 million tonnes of oil (479.6 million barrels), 4.73 million tonnes of natural gas liquids and 1.42 billion cubic metres of natural gas[13] that will last until 2020 and 2025 respectively.
Oil is also transported to Wytch Farm for processing from two smaller Dorset oilfields, Wareham (discovered in 1964) by pipeline and Kimmeridge Oil Field (discovered in 1959) by road.
Oil is piped about 91 kilometres (57 mi) from Wytch Farm via Fawley to a terminal on the far side of Southampton Water at Hamble, for export by tanker. Natural Gas (methane) is piped to Sopley, north of Christchurch, for use in the national domestic gas supply network. Smaller quantities of liquefied petroleum gas are transported by road. A rail terminal at Furzebrook connecting to the Swanage Railway between Corfe Castle and Wareham is now closed and mothballed.
The oil-shale bings of West Lothian[]
The area around Livingston was previously an important shale oil area, the world's first oil boom occurred in West Lothian. This was based on oil extracted from shale, and by 1870 over 3 million tons of shale were being mined each year in the area around Livingston. Output declined with the discovery of liquid oil reserves around the world in the early 1900s, but shale mining only finally ceased in 1962. The "bings" that characterise oil shale mining in West Lothian have largely been flattened. West Lothian Oil-Shale Formation.
In 1898 Livingston had several houses, a Church of Scotland church, a United Free church and a school. Around 1 mile north of Livingston there was a railway station in a settlement called Livingston Station which is now part of Deans.
There were major local outcrops of cannel coal, Westphalian coal measures and torbanite, all of which had been in informal use as a domestic and industrial fuel since the 16th century. The Westphalian coal measures extend west through Lanarkshire and were mined their and in there maller outcrops in the Lothians and Fife. There were also Millstone grit series, the source of fireclay; Carboniferous limestone series formed of narrow bands of limestone and sandstone, calcareous mudstone (‘marl’) and tuffaceous mudstones. They were of use to Hanoverian and Victoran industries in the Livingstone area.
In 1847 the Scottish chemist James Young prepared "lighting oil," lubricating oil and wax from cannel coal and since 1862 from torbanite. In 1850 he patented the process of retorting and refining shale oil and purifying paraffin wax from it. Commercial scale shale oil extraction from lamosite started in 1859 by Robert Bell in Broxburn, West Lothian. In After expiring of Young's patent in 1862 many small shale oil works were opened. By 1865, there were about 120 shale oil works in Scotland. In 1866 Young established Young's Paraffin Light and Mineral Oil Company at Addiewell. Other notable shale oil companies were the Broxburn Oil Company established in 1878 and the Pumpherston Oil Company established in 1892.
The oil shale industry expanded immediately before World War I because of limited access to conventional petroleum resources and the mass production of automobiles and trucks, which accompanied an increase in gasoline consumption. Oil shale production in Scotland peaked in 1910–1912 with more than three million tonnes. That time Scottish shale oil industry contributed 2% of global oil production. After that, production declined with exception of the period of World War II. In 1919, five survived shale oil companies (Young's Paraffin Light & Mineral Oil Company, Broxburn Oil Company, Pumpherston Shale Oil Company, Oakbank Oil Company, and James Ross & Company Philpstoun Oil Works), were merged into Scottish Oils, a subsidiary of Anglo-Persian Oil Company.
Scottish chemist and geologist, James “Paraffin” Young, took out British patent number 13292 in 1850 and later an American patent to register a new process for retorting and refining oil, and subsequently purifying paraffin wax from it. He also opened the original Bathgate Chemical Works paraffin works (the world’s first formal oil refinery) in Bathgate, West Lothian during 1851. It processed Cannel (parrot) coal (discovered by Young, near Broxburn and West Calder in 1858) for paraffin. The Canadian chemist and geolagist Abraham Pineo Gesner also produced kerosene from New Brunswick, Nova Scotia and New York oil shale and coal in 1846.
The mineral oils were processed on an industrial scale, competed and continued in operation until 1962 with a estimated that 164 million tons of oil shale was mined in the life of the industry.
The remaining 19 shale bings of West Lothian are the large, 30 to 90 metre high spoil heaps made from the waste material from an industrial process to retort crude oil (paraffin) from deep mined oil-bearing shale. They have been 'unworked' (not used) from between 87 and 43 years.
The La Brea Tar Pits[]

The Tar Pits in 1910; oil derricks can be seen in the background
The La Brea Tar Pits are a group of tar pits around which Hancock Park was formed in urban Los Angeles. Natural asphalt (also called asphaltum, bitumen, pitch or tar—brea in Spanish) has seeped up from the ground in this area for tens of thousands of years. The tar is often covered with dust, leaves, or water. Over many centuries, the bones of animals that were trapped in the tar were preserved. The George C. Page Museum is dedicated to researching the tar pits and displaying specimens from the animals that died there. The La Brea Tar Pits is a registered National Natural Landmark.
The modern name is an example of a tautological place name; "the La Brea Tar Pits" literally means "the the tar tar pits."
The La Brea Tar Pits and Hancock Park are situated within what was once the Mexican land grant of Rancho La Brea, now part of urban Los Angeles in the Miracle Mile district, adjacent to the Los Angeles County Museum of Art and the Craft and Folk Art Museum.
The tar pits visible today are actually from human excavation. The lake pit was originally an asphalt mine. The other pits visible today were produced between 1913 and 1915, when over 100 pits were excavated in search of large mammal bones. Various combinations of asphaltum and water have since filled in these holes. Normally, the asphalt appears in vents, hardening as it oozes out, to form stubby mounds. These can be seen in several areas of the park.
Tar pits are composed of heavy oil fractions called gilsonite, which seeped from the Earth as oil. In Hancock Park, crude oil seeps up along the 6th Street Fault from the Salt Lake Oil Field, which underlies much of the Fairfax District north of the park. The oil reaches the surface and forms pools at several locations in the park, becoming asphalt as the lighter fractions of the petroleum biodegrade or evaporate.
This seepage has been happening for tens of thousands of years. From time to time, the asphalt would form a deposit thick enough to trap animals, and the surface would be covered with layers of water, dust, or leaves. Animals would wander in, become trapped, and eventually die. Predators would enter to eat the trapped animals and also become stuck.
As the bones of dead animals sink into the asphalt, it soaks into them, turning them a dark-brown or black color. Lighter fractions of petroleum evaporate from the asphalt, leaving a more solid substance, which encases the bones. Dramatic fossils of large mammals have been extricated from the tar, but the asphalt also preserves microfossils: wood and plant remnants, rodent bones, insects, mollusks, dust, seeds, leaves, and even pollen grains. Examples of some of these are on display in the George C. Page museum.
Radiometric dating of preserved wood and bones has given an age of 38,000 years for the oldest known material from the La Brea seeps. The pits still ensnare organisms today, so most of the pits are fenced to protect humans and animals.
The Native American Chumash and Tongva people living in the area built boats unlike any others in North America prior to contact by settlers. Pulling fallen Northern California redwood trunks and pieces of driftwood from the Santa Barbara Channel, their ancestors learned to seal the cracks between the boards of the large wooden plank canoes by using the natural resource of tar. This innovative form of transportation allowed access up and down the coastline and to the Channel Islands.
The Portolá expedition, a group of Spanish explorers led by Gaspar de Portolá, made the first written record of the tar pits in 1769. Father Juan Crespí wrote,
While crossing the basin the scouts reported having seen some geysers of tar issuing from the ground like springs; it boils up molten, and the water runs to one side and the tar to the other. The scouts reported that they had come across many of these springs and had seen large swamps of them, enough, they said, to caulk many vessels. We were not so lucky ourselves as to see these tar geysers, much though we wished it; as it was some distance out of the way we were to take, the Governor [Portolá] did not want us to go past them. We christened them Los Volcanes de Brea [the Tar Volcanoes].
Harrison Rogers, who accompanied Jedediah Smith on his 1826 expedition to California, was shown a piece of the solidified asphalt while at Mission San Gabriel, and noted in his daybook (journal) that "The Citizens of the Country make great use of it to pitch the roofs of their houses".
For some years, tar-covered bones were found on the Rancho La Brea property but were not initially recognized as fossils because the ranch had lost various animals, including horses, cattle, dogs, and even camels, whose bones closely resemble several of the fossil species. The original Rancho La Brea land grant stipulated that the tar pits be open to the public for the use of the local Pueblo. Initially, they mistook the bones in the pits for the remains of pronghorn antelope (Antilocapra americana) or cattle that had become mired.
Union Oil geologist W. W. Orcutt is credited, in 1901, with first recognizing that fossilized prehistoric animal bones were preserved in pools of asphalt on the Hancock Ranch. In commemoration of Orcutt's initial discovery, paleontologists named the La Brea coyote (Canis orcutti) in his honor.
Columbian mammoth skeleton from the tar pits displayed in the Page museum Contemporary excavations of the bones started in 1913–1915. In the 1940s and 1950s, public excitement was generated by the preparation of previously recovered large mammal bones. Subsequent study demonstrated the fossil vertebrate material was well preserved, with little evidence of bacterial degradation of bone protein.
Methane gas escapes from the tar pits, causing bubbles that make the asphalt appear to boil. Asphalt and methane appear under surrounding buildings and require special operations for removal to prevent the weakening of building foundations.
In 2007, researchers from UC Riverside discovered that the bubbles were caused by hardy forms of bacteria embedded in the natural asphalt. After consuming petroleum, the bacteria release methane. "Of the bacteria sampled, about 200 to 300 were previously unknown species."
Synthetic crude[]
Synthetic crude oil, also known as syncrude, is the output from a bitumen upgrader facility used in connection with oil sand production in Canada. Bituminous sands are mined using enormous (100 ton capacity) power shovels and loaded into even larger (400 ton capacity) dump trucks for movement to an upgrading facility. The process used to extract the bitumen from the sand is a hot water process originally developed by Dr. Karl Clark of the University of Alberta during the 1920s. After extraction from the sand, the bitumen is fed into a bitumen upgrader which converts it into a light crude oil equivalent. This synthetic substance is fluid enough to be transferred through conventional oil pipelines and can be fed into conventional oil refineries without any further treatment. By 2015 Canadian bitumen upgraders were producing over 1 million barrels (160×103 m3) per day of synthetic crude oil, of which 75% was exported to oil refineries in the United States.
In Alberta, five bitumen upgraders produce synthetic crude oil and a variety of other products: The Suncor Energy upgrader near Fort McMurray, Alberta produces synthetic crude oil plus diesel fuel; the Syncrude Canada, Canadian Natural Resources, and Nexen upgraders near Fort McMurray produce synthetic crude oil; and the Shell Scotford Upgrader near Edmonton produces synthetic crude oil plus an intermediate feedstock for the nearby Shell Oil Refinery. A sixth upgrader, under construction in 2015 near Redwater, Alberta, will upgrade half of its crude bitumen directly to diesel fuel, with the remainder of the output being sold as feedstock to nearby oil refineries and petrochemical plants.
Synthetic crude is the output from a bitumen/extra heavy oil upgrader facility used in connection with oil sand production. It may also refer to shale oil, an output from an oil shale pyrolysis. The properties of the synthetic crude depend on the processes used in the upgrading. Typically, it is low in sulfur and has an API gravity of around 30. It is also known as "upgraded crude".
Synthetic crude is an intermediate product produced when an extra-heavy or unconventional oil source is upgraded into a transportable form. Synthetic crude is then shipped to oil refineries where it is further upgraded into finished products. Synthetic crude may also be mixed, as a diluent, with heavy oil to create synbit. Synbit is more viscous than synthetic crude, but can be a less expensive alternative for transporting heavy oil to a conventional refinery.
Syncrude Canada, Suncor Energy Inc., and Canadian Natural Resources Limited are the three largest worldwide producers of synthetic crude with a cumulative production of approximately 600,000 barrels per day (95,000 m3/d). The NewGrade Energy Upgrader became operational in 1988, and was the first upgrader in Canada, now part of the CCRL Refinery Complex.
Syncrude[]

Syncrude's Mildred Lake mine site and plant. In this picture of Syncrude's base mine. The yellow structures are the bases of pyramids made of sulphur - it is not economical for Syncrude to sell the sulphur so it stockpiles it instead. Behind that is the tailings pond, held in by what is recognized as the largest dam in the world. The extraction plant is just to the right of this photograph and most of the mine is to the left.
Overview[]
Syncrude Canada Ltd. is one of the world's largest producers of synthetic crude oil from oil sands and the largest single source producer in Canada. It is located just outside Fort McMurray in the Athabasca Oil Sands, and has a nameplate capacity of 350,000 barrels per day (56,000 m3/d) of oil, equivalent to about 13% of Canada's consumption. It has approximately 5.1 billion barrels (810,000,000 m3) of proven and probable reserves (11.9 billion when including contingent and prospective resources) situated on 8 leases over 3 contiguous sites. Including fully realized prospective reserves, current production capacity could be sustained for well over 90 years.
The company is a joint venture between five partners. As a result, Syncrude is not traded directly, but rather through the individual owners. As of June 2016, the partners (by percentage): Suncor Energy (53.74%), Imperial Oil (25%), Sinopec (9.03%), Nexen (7.23%), and Mocal Energy (a subsidiary of Nippon Oil Exploration) (5%). Because of Nexen's subsequent takeover by CNOOC, over 16% of the shares in Syncrude are controlled by State Owned Enterprises (SOE).
The ownership board must approve all annual operating budgets and proposed capital spending projects, and are required to provide the funding for said activities based on their ownership share.
Corporate History[]
Syncrude was formed as a research consortium in 1964. Construction at the Syncrude site began in 1973, and it officially opened in 1978. Starting in 1996, Syncrude has been expanding its operations. Between 1996 and 1999, the original mine was expanded and the plant was "debottlenecked", increasing production from 73.5 million barrels (11,690,000 m3) per year in 1996 to 81.4 million in 1999. The total cost of this stage of expansion was $470 million. Between 1998 and 2001, a new mine, Aurora, was opened 35 km north of the original site, and further debottlenecking was undertaken. Production started in Aurora in July 2001. Syncrude's production increased to 90 million barrels (14,000,000 m3) per year by the end of 2001. Total cost for this stage was $1 billion.
A third stage of expansion was undertaken between 2001 and 2006, in which a second train at the Aurora line came online and the Mildred Lake upgrader was expanded. The expansion added 100,000 barrels per day (16,000 m3/d) to Syncrude's production (36.5 million barrels (5,800,000 m3) a year assuming this is average). The cost was $8.4 billion, a substantial cost overrun over the original estimate of $5.7 billion.
On April 12, 2010, ConocoPhillips agreed to sell its share to Sinopec, a Chinese state-owned oil company. The sale, for $4.65 billion, was completed on June 25, 2010.
A 183 m (600 ft) smokestack is located at the facility which is the second tallest in western Canada.
In April 2016, Suncor announced that they had reached a $937-million deal to acquire Murphy Oil Corp.'s five per cent stake in the Syncrude project north of Fort McMurray, Alta. This follows the hostile takeover of Canadian Oil Sands less than a year ago, and will increase its interest in Syncrude from just under 49 per cent to nearly 54 per cent, making it the majority shareholder of the project.
The 2016 Fort McMurray wildfire forced a complete shutdown of Syncrude's facilities, a few of which were damaged or destroyed.
Pollution controversy[]
Air releases of combined gases without volatile organic compounds (VOCs) by Syncrude Canada in 2005 were 129,741,321 (kg) in total, including ammonia (4,302,361 kg), sulphuric acid (1,129,425 kg), xylene (501,461 kg), etc. The company was also ranked as having the seventh highest air releases of combined gases (without VOC) in Canada in 2005. Syncrude's Mildred Lake Plant Site is the largest greenhouse gas emitter in Canada emitting 12,359,420 tonnes of CO2 equivalent in 2012.
Corporate stats[]
- Name- Syncrude Canada Ltd.
- Corporate type- Joint Venture
- Industry- Oil and Gas
- Founded- December 1964
- Headquarters- Fort McMurray, Alberta
- Products- Petroleum
- Number of employees- 5,600 (2009)
- Website- www.syncrude.ca
Locations[]
Oil sand locations[]
- Canada
- Melville Island
- Baffin Island
- Athabasca oil sands
- Northern Sesckatuain
- Northern Manitoba
- South eastern British Colombia
- Southern Yukon
- Venezuela
- USA
- Utah
- Southern Alaska
- California
- Louisiana
- Montana
- Texas
- New York
- Connecticut
- Ohio
- Illinois
- Florida
- N. Dakota
- Australia
- Uganda
- Kenya
- Burundi
- Rwanda
- Tanzania
- Greenland
- France
- Poland
- Greece
- Bulgaria
- Iceland
- Sweden
- Norway
- Finland
- Spain
- Italy
- Bosnia
- Madagascar
- S. Africa
- Barbados
- Bahamas
- Cuba
- Oman
- Argentina
- China
- Junggar Basin
- Tarim Basin
- Turpan Basin
- Qaidam Basin
- Ordos Basin
- Scichuan Basin
- Jianghan Basin
- Subie Basin
- Songlaio Basin
- Pearl River Mouth Basin
- South China Basin
- Yangtze Basin
- Kazakhstan
- To the north of the Caspian Sea
- In the north of the Caspian Sea
- Russia
- Tunguska Basin
- Tatarstan
- Dagestan
- Central Urals Mountains
- Sakhalin Island
- Mongolia
- Estonia
- Latvia
- Lithuania
- Belorussian
- Ukraine
- Nova-Russia
- Afghanistan
- Pakistan
- Tajikistan
- Turkmenistan
- Uzbekistan
- Azerbaijan
- Armenian
- Georgia
- Moldavia
- Transdentstria
- Nagorno-Karabach
- N. Osetia
- Chechnya
- Albania,
- Trinidad
- N. Korea
- Romania
- Serbia
- Bosnia
- Hungary
- Croatia
- Macedonia
- Greece
- Slovenia
- Italy
- Poland
- Madagascar
- Tsimiroro
- Bemolanga
- Republic of the Congo
- Brazil
- Guyana
- Colombia
- Equator
- Peru
- Nigeria
- India
- Iran
- Ghana
- Egypt
- North Sudan
- South Sudan
- Mexico
- Saudi Arabia
- Kuwait
- Iraq
- Turkey
- Jordan
- Syria
- Vietnam
- Burma
- UK
- Mid Wales coastline
- Dorset
- Lankashire,
- Durhamshire,
- E. Yorkshire
- Oxfordshire
- Berkshire
- Surrey
- Essex
- North Norfolk
- South Lincolnshire
- Bukinghamshire
- Derbyshire
- Staffordshire
- Devon
- Sterlingshire
- Cornwall
- Vale of Glamorgan
- Monmouthshire
- Tayside
- Northumbrian coast
- Cumbrian coast
- Dumfress-shire
- Inverness-shire.
- Easter Ross
- Malaysia
- Indonesia
- Thailand
- Uganda
- Niger
- Kenya
- Tanzania
- Senegal
- Mozambique
- Somalia
- Ethiopia
- DRC
- Congo (Brazaville)
- Angola
- Mexico
- Bolivia
- Paraguay
- Uruguay
- Brazil
- Pakistan
- Morocco
- Libya
- Algeria
- Slovakia
- Austria
- Luxembourg
- Switzerland
- Liechtenstein
- France
- Belgian
- Netherlands
- Denmark
- Hungary
- Czech Republic
In May 2008, the Italian oil company Eni announced a project to develop a small oil sands deposit in the Republic of the Congo. Production is scheduled to commence in 2014 and is estimated to eventually yield a total of 40,000 bbl/d (6,400 m3/d). The reserves are estimated between 0.5 and 2.5 Gbbl (79×106 and 397×106 m3).
Methods for extraction include Cold heavy oil production with sand, steam assisted gravity drainage, steam injection, vapor extraction, Toe-to-Heel Air Injection (THAI), and open-pit mining for extremely sandy and oil-rich deposits.
Heavy crude oil (or extra heavy crude oil) locations[]
Heavy oil is asphaltic and contains asphaltenes and resins. It is "heavy" (dense and viscous) due to the high ratio of aromatics and naphthenes to linear alkanes and high amounts of NSO's (nitrogen, sulfur, oxygen and heavy metals). Heavy oil has a higher percentage of compounds with over 60 carbon atoms and hence a high boiling point and molecular weight. For example, the viscosity of Venezuela's Orinoco extra-heavy crude oil lies in the range 1000–5000 cP (1–5 Pa·s), while Canadian extra-heavy crude has a viscosity in the range 5000–10,000 cP (5–10 Pa·s), about the same as molasses, and higher (up to 100,000 cP or 100 Pa·s for the most viscous commercially exploitable deposits).
Most geologists agree that crude becomes ‘heavy’ as a result of biodegradation, in which lighter ends are preferentially consumed by bacterial activity in the reservoir, leaving heavier hydrocarbons behind. This hypothesis leans heavily on the techniques of petroleum geochemistry. Poor geologic reservoir sealing exposes the hydrocarbon to surface contaminants, including organic life (such as bacteria) and contributes to this process.
Heavy oils can be found in shallow, young reservoirs, with rocks from the Pleistocene, Pliocene, and Miocene (younger than 25 million years). In some cases, it can also be found in older Cretaceous, Mississippian, and Devonian reservoirs. These reservoirs tend to be poorly sealed, resulting in heavy oil and oil-sands.
- Canada
- Alberta
- Saskatchewan
- United States
- California
- Arizona
- Wyoming
- Texas
- Utah
- Mexico
- Venezuela
- Colombia
- Ecuador
- Kuwait
- Saudi Arabia
- East Timor
- Indoniaia
- West Timor
- Coastal Borneo
- Chad
- Angola
- Madagascar
Oil shales locations[]
Major oil shale deposits are located in the Democratic Republic of Congo (equal to 14.31 billion metric tons of shale oil) and Morocco (12.3 billion metric tons or 8.16 billion metric tons of shale oil). Deposits in Congo are not properly explored yet. In Morocco, oil shale deposits have been identified at ten localities with the largest deposits in Tarfaya and Timahdite. Although reserves in Tarfaya and Timahdit are well explored, the commercial exploitation has not started yet and only a limited program of laboratory and pilot-plant research has been undertaken. There are also oil shale reserves in Egypt, South Africa, Madagascar, and Nigeria. The main deposits of Egypt are located in Safaga-Al-Qusayr and Abu Tartour areas.
Major oil shale deposits are located in China, which has an estimated total of 32 billion metric tons, of which 4.4 billion metric tons are technically exploitable and economically feasible. The principal Chinese oil shale deposits and production lie in Fushun and Liaoning; others are located in Maoming in Guangdong, Huadian in Jilin, Heilongjiang, and Shandong. Professor Alan R. Carroll of University of Wisconsin–Madison estimates that Upper Permian lacustrine oil shale deposits of northwest China, absent from previous global oil shale assessments, are comparable to the Green River Formation.
In addition to China, major deposits are located in Thailand (18.7 billion metric tons), Pakistan (227 billion metric tons)of which 9.1 billion metric tons are technically exploitable and economically feasible;, Kazakhstan (several deposits; major deposit at Kenderlyk Field with 4 billion metric tons), and Turkey (2.2 billion metric tons). Thailand's oil shale deposits are near Mae Sot, Tak Province, and at Li, Lamphun Province. Deposits in Turkey are found mainly in middle and western Anatolia. According to some reports, also Uzbekistan has major oil shale deposits of 47 billion metric tons, mainly located at Sangruntau but also at Baysun, Jam, Urtabulak, Aktau, Uchkyr and Kulbeshkak. Smaller oil shale reserves have also been found in India, Turkmenistan, Myanmar, Armenia, and Mongolia.
The biggest oil shale reserves in Europe are located in Russia (equal to 35.47 billion metric tons of shale oil). Major deposits are located in the Volga-Petchyorsk province and in the Baltic Oil Shale Basin. Other major oil shale deposits in Europe are located in Italy (10.45 billion metric tons of shale oil), Estonia (2.49 billion metric tons of shale oil), France (1 billion metric tons of shale oil), Belarus (1 billion metric tons of shale oil), Sweden (875 million metric tons of shale oil), Ukraine (600 million metric tons of shale oil) and the United Kingdom (500 million metric tons of shale oil). There are oil shale reserves also in Germany, Luxembourg, Spain, Bulgaria, Hungary, Poland, Serbia, Austria, Albania, and Romania.
Significant oil shale deposits are located in Jordan (5.242 billion metric tons of shale oil or 65 billion metric tons of oil shale) and Israel (550 million metric tons of shale oil or 6.5 billion metric tons of oil shale). Jordanian oil shales are high quality, comparable to western US oil shale, although their sulfur content is high. The best-explored deposits are El Lajjun, Sultani, and the Juref ed Darawish are located in west-central Jordan, while the Yarmouk deposit, close to its northern border, extends into Syria. Most of Israel's deposits are located in the Rotem Basin region of the northern Negev desert near the Dead Sea. Israeli oil shale is relatively low in heating value and oil yield.
Oil shale from the Mahogany Zone of the Green River Formation, Colorado. Weathered surface on right; fresh surface on left. At 301 billion metric tons, the oil shale deposits in the United States are easily the largest in the world. There are two major deposits: the eastern US deposits, in Devonian-Mississippian shales, cover 250,000 square miles (650,000 km2); the western US deposits of the Green River Formation in Colorado, Wyoming, and Utah, are among the richest oil shale deposits in the world. n Canada 19 deposits have been identified. The best-examined deposits are in Nova Scotia and New Brunswick.
Australia's oil shale resource is estimated at about 58 billion metric tons or 4.531 billion metric tons of shale oil, of which about 24 billion barrels (3.8 billion cubic metres) is recoverable. The deposits are located in the eastern and southern states with the biggest potential in the eastern Queensland deposits. Oil shale has also been found in New Zealand.
Brazil has the world's second-largest known oil shale resources (the Irati shale and lacustrine deposits) and is currently the world's second largest shale oil producer, after Estonia. Oil shale resources occur in São Mateus do Sul, Paraná, and in Vale do Paraíba. Brazil has developed the world’s largest surface oil shale pyrolysis retort at Petrosix, with a 11-meter (36 ft)-diameter vertical shaft. Brazilian production in 1999 was about 200,000 metric tons. Small resources are also found in Argentina, Chile, Paraguay, Peru, Uruguay, and Venezuela.
- Serbia
- Croatia
- Slovenia
- Macedonia
- Austria
- Switzerland
- Hungary
- Slovakia
- Hungray
- Georgia
- Armenia
- Uganda
- S. Sudan
- N. Sudan
- Ethiopia
- Zambia
- Malawi
- S. Africa
- Botswana
- Somalia
- Kenya
- Tanzania
- Brazil,
- China,
- Estonia
- Germany
- Russia.
- Estonia,
- Israel,
- Romania
- Jordan
- Egypt
- Canada
- Turkey
- Palestine
- Syria
- Lebanon
- Azerbaijan
- Iran
- Iraqi Kurdistan
- Albania
- Greece
- DRC
- Peru
- USA
- New Zealand
- Mongolia
- Kazakhstan
- Australia
- France
- Belgium
- Sweden
- Greenland
- Indonesia
- Estonia
- Latvia
- Lituania
- Poland
- Sweden
- United States,
- Australia,
- Sweden
- Estonia,
- Jordan,
- France,
- Germany,
- Brazil,
- Mongolia
- Russia.
- Tunguska Basin
- Central Ural Mountains
- Tartarstan
- Volga-Petchyorsk province
- Baltic Oil Shale Basin.
- Ukraine
- Brazil
- Romania
- Moldova
- Transdenstria
- Ukraine
- Belorussian
- Argentina
- Paraguay
- Brazil
- Bolivia
- Colombia
- Valenzuela
- Mexico
- Guyana
- Suriname
- Aruba
- Trinidad and Tobago
- French Gueania
- Democratic Republic of Congo
- Morocco
- Republic of Congo (Brazaville)
- Egypt,
- South Africa,
- Madagascar,
- Nigeria.
- China,
- Fushun
- Liaoning;
- Guangdong,
- Jilin,
- Heilongjiang,
- Shandong.
- Thailand
- Pakistan
- Kazakhstan
- Turkey
- India,
- Turkmenistan,
- Myanmar,
- Armenia,
- Mongolia.
- Estonia
- Belarus
- Sweden
- Ukraine
- United Kingdom
- Germany,
- Luxembourg,
- Spain,
- Bulgaria,
- Hungary,
- Poland,
- Serbia,
- Austria,
- Albania,
- Romania.
- Jordan
- Israel
- Jordan
- Syria.
- USA
- Colorado.
- Mississippi
- Wyoming,
- Utah,
- Canada
- Nova Scotia
- New Brunswick.
- Australia
- New Zealand.
- Brazil
- Argentina,
- Chile,
- Paraguay,
- Peru,
- Uruguay,
- Egypt,
- South Africa,
- Nigeria.
- Venezuela.
The largest deposits in the world occur in the United States in the Green River Formation, which covers portions of Colorado, Utah, and Wyoming; about 70% of this resource lies on land owned or managed by the United States federal government. Deposits in the United States constitute 62% of world resources; together, the United States, Russia and Brazil account for 86% of the world's resources in terms of shale-oil content. These figures remain tentative, with exploration or analysis of several deposits still outstanding. Professor Alan R. Carroll of University of Wisconsin–Madison regards the Upper Permian lacustrine oil-shale deposits of northwest China, absent from previous global oil shale assessments, as comparable in size to the Green River Formation.
According to the World Energy Council, in 2008 the total production of shale oil from oil shale was 930,000 tonnes, equal to 17,700 barrels per day (2,810 m3/d), of which China produced 375,000 tonnes, Estonia 355,000 tonnes, and Brazil 200,000 tonnes. In comparison, production of the conventional oil and natural gas liquids in 2008 amounted 3.95 billion tonnes or 82.1 million barrels per day (13.1×106 m3/d).
Gilsonite/bitumen stone locations[]
Firms like Zista.co mine the reserve in Uintah Basin of Utah and Colorado.
- USA
- Colorado.
- Wyoming,
- Nevada,
- California,
- Oregon
- Palestine.
- Israel.
Natural a tar (AKA- pitch) pit locations[]

Gas bubble slowly emerging at La Brea Tar Pits.
The tar pits visible today are actually from human excavation. The lake pit was originally an asphalt mine. The other pits visible today were produced between 1913 and 1915, when over 100 pits were excavated in search of large mammal bones. Various combinations of asphaltum and water have since filled in these holes. Normally, the asphalt appears in vents, hardening as it oozes out, to form stubby mounds. These can be seen in several areas of the park.
They are located in:
- Trinidad and Tobago
- Estado Sucre, Venezuela
- California, USA
- Hīt, Iraq
- Baku, Azerbaijan
- Komi Region, Russia
- Formerly at the Biblical 'Lake Asphaltitis' near the Dead Sea. It was also used as a alternate for the Dead Sea. The name for both was fist coined by Titus Flavius Josephus.
What is 'tight oil' locations[]

Shown are conceptual illustrations of types of oil and gas wells. A vertical well is producing from a conventional oil and gas deposit (right). Also shown are wells producing from unconventional formations: a vertical coalbed methane well (second from right); a horizontal well producing from a shale formation (center); and a well producing from a tight sand formation (left).
Tight oil (also known as shale oil or light tight oil, abbreviated LTO) is petroleum that consists of light crude oil contained in petroleum-bearing formations of low permeability, often shale or tight sandstone. Economic production from tight oil formations requires the same hydraulic fracturing and often uses the same horizontal well technology used in the production of shale gas. It should not be confused with oil shale, which is shale rich in kerogen, or shale oil, which is oil produced from oil shales.
Following are estimates of technically recoverable volumes of tight oil associated with shale formations, made by the US Energy Information Administration in 2013. Not all oil which is technically recoverable may be economically recoverable at current or anticipated prices.
- Russia: 75 billion barrels
- United States: 48 to 58 billion barrels
- China: 32 billion barrels
- Argentina: 27 billion barrels
- Libya: 26 billion barrels
- Venezuela: 13 billion barrels
- Mexico: 13 billion barrels
- Pakistan: 9 billion barrels
- Canada: 9 billion barrels
- Indonesia: 8 billion barrels
- World Total 335 to 345 billion barrels
- A Australian private oil company announced that it had discovered tight oil in shale of the Arckaringa Basin, which they estimated at 3.5 to 223 billion barrels.
Cannel coal locations[]
- USA
- New York,
- Pennsylvania,
- Ohio,
- Kentucky,
- West Virginia
- UK
- Livingstone
- Kirkcaldy
- Musselburgh
- Durhamshire
- Greater Manchester
- Southern Lancashire
- Ireland
Kukersite locations[]
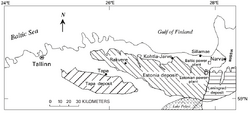
Location of the kukersite deposits within the Baltic Oil Shale Basin in northern Estonia and Russia.
- Estonian
- Russia
- Leningrad
- Veimarn
- Chudovo–Babinskoe
- USA
- Michigan
- Illinois
- Wisconsin
- North Dakota
- Oklahoma
- Australia
- Amadeus basins
- Canning basins
- UK
- Orkneys
- Shetlands
- Outer Hebradies
- Sky
- Assynt
- Sutherland coastline
- Caithness's coastline
- Finland
- Faeroe Islands
- North Sea's sea bed-
- UK oil\gas drilling zones's sea bed.
- Norwegian oil\gas drilling zones's sea bed.
- Dutch oil\gas drilling zones's sea bed.
- German oil\gas drilling zones's sea bed.
- Danish oil\gas drilling zones's sea bed.
The Baltic Oil Shale Basin covers about 3,000 to 5,000 square kilometres (1,200 to 1,900 sq mi). Main kukersite deposits are Estonian and Tapa deposits in Estonia, and Leningrad deposit in Russia (also known as Gdov or Oudova deposit). Other occurrences in Russia are Veimarn and Chudovo–Babinskoe deposits. The Estonian deposit, which covers about 2,000 square kilometres (770 sq mi), is exploited industrially; the Tapa deposit is not accounted as reserves due its lower value which makes its extraction economically inexpedient. The Leningrad deposit was exploited industrially but operations have ceased.
Some in both Israel and Palestine have regarded it on occasion as a possible energy source during the late 1970s and early 1990s. The USSR, Brazil, China, the GDR, the FRG, Russia and Estonia regard it as a handy local source of oil that can help maintain thier energy industry.
Asphalt/bitumen locations[]

Natural asphalt/bitumen from the Dead Sea.
They are located in:
- France
- Philippines
- Canada
- Trinidad and Tobago
- Venezuela
- USA
- Russia
- Greece
- Turkey
- Syria
- Iraq
- Lebanon
- Iran
- Azerbaijan
- Palestine
- Jordan
- Israel
- Saudi Arabia
- Oman
- Yemen
- China
- India
- Australia
- S. Africa
- Kuwait
- Egypt
- N. Sudan
- S. Sudan
- Libya
Extraction methods[]
Shale oil extraction[]
Shale oil extraction is an industrial process for unconventional oil production. This process converts kerogen in oil shale into shale oil by pyrolysis, hydrogenation, or thermal dissolution. The resultant shale oil is used as fuel oil or upgraded to meet refinery feedstock specifications by adding hydrogen and removing sulfur and nitrogen impurities.
Shale oil extraction is usually performed above ground (ex situ processing) by mining the oil shale and then treating it in processing facilities. Other modern technologies perform the processing underground (on-site or in situ processing) by applying heat and extracting the oil via oil wells.
The earliest description of the process dates to the 10th century. In 1684, Great Britain granted the first formal extraction process patent. Extraction industries and innovations became widespread during the 19th century. The industry shrank in the mid-20th century following the discovery of large reserves of conventional oil, but high petroleum prices at the beginning of the 21st century have led to renewed interest, accompanied by the development and testing of newer technologies.
As of 2010, major long-standing extraction industries are operating in Estonia, Brazil, and China. Its economic viability usually requires a lack of locally available crude oil. National energy security issues have also played a role in its development. Critics of shale oil extraction pose questions about environmental management issues, such as waste disposal, extensive water use, waste water management, and air pollution.
Internal combustion[]
Internal combustion technologies burn materials (typically char and oil shale gas) within a vertical shaft retort to supply heat for pyrolysis. Typically raw oil shale particles between 12 millimetres (0.5 in) and 75 millimetres (3.0 in) in size are fed into the top of the retort and are heated by the rising hot gases, which pass through the descending oil shale, thereby causing decomposition of the kerogen at about 500 °C (932 °F) . Shale oil mist, evolved gases and cooled combustion gases are removed from the top of the retort then moved to separation equipment. Condensed shale oil is collected, while non-condensable gas is recycled and used to carry heat up the retort. In the lower part of the retort, air is injected for the combustion which heats the spent oil shale and gases to between 700 °C (1,292 °F) and 900 °C (1,650 °F). Cold recycled gas may enter the bottom of the retort to cool the shale ash. The Union A and Superior Direct processes depart from this pattern. In the Union A process, oil shale is fed through the bottom of the retort and a pump moves it upward. In the Superior Direct process, oil shale is processed in a horizontal, segmented, doughnut-shaped traveling-grate retort.
Internal combustion technologies such as the Paraho Direct are thermally efficient, since combustion of char on the spent shale and heat recovered from the shale ash and evolved gases can provide all the heat requirements of the retort. These technologies can achieve 80-90% of Fischer assay yield. Two well-established shale oil industries use internal combustion technologies: Kiviter process facilities have been operated continuously in Estonia since the 1920s, and a number of Chinese companies operate Fushun process facilities.
Common drawbacks of internal combustion technologies are that the combustible oil shale gas is diluted by combustion gases and particles smaller than 10 millimeters (0.4 in) can not be processed. Uneven distribution of gas across the retort can result in blockages when hot spots cause particles to fuse or disintegrate.
Hot recycled solids[]
Hot recycled solids technologies deliver heat to the oil shale by recycling hot solid particles—typically oil shale ash. These technologies usually employ rotating kiln or fluidized bed retorts, fed by fine oil shale particles generally having a diameter of less than 10 millimeters (0.4 in); some technologies use particles even smaller than 2.5 millimeters (0.10 in). The recycled particles are heated in a separate chamber or vessel to about 800 °C (1,470 °F) and then mixed with the raw oil shale to cause the shale to decompose at about 500 °C (932 °F). Oil vapour and shale oil gas are separated from the solids and cooled to condense and collect the oil. Heat recovered from the combustion gases and shale ash may be used to dry and preheat the raw oil shale before it is mixed with the hot recycle solids.
In the Galoter and Enefit processes, the spent oil shale is burnt in a separate furnace and the resulting hot ash is separated from the combustion gas and mixed with oil shale particles in a rotating kiln. Combustion gases from the furnace are used to dry the oil shale in a dryer before mixing with hot ash. The TOSCO II process uses ceramic balls instead of shale ash as the hot recycled solids. The distinguishing feature of the Alberta Taciuk Process (ATP) is that the entire process occurs in a single rotating multi–chamber horizontal vessel.
Because the hot recycle solids are heated in a separate furnace, the oil shale gas from these technologies is not diluted with combustion exhaust gas. Another advantage is that there is no limit on the smallest particles that the retort can process, thus allowing all the crushed feed to be used. One disadvantge is that more water is used to handle the resulting finer shale ash.
It is a horizontal cylinder 8.2 meters (27 ft) high and 62.5 meters (205 ft) wide. The raw oil shale is fed from the right side and it moves to a section where it is dried and preheated by hot oil shale ash. The temperature in this section is around 250 °C (482 °F). At the same time, the raw oil shale in this section serves to cool the resultant oil shale ash before its removal. In the retorting section, the temperature is around 500 °C (932 °F). Oil vapors are removed through the vapor tube. The spent oil shale is again heated in the combustion section to a temperature of 750 °C (1,380 °F) and ash is generated. The ash is then sent to the retorting section as a heat carrier, or to the cooling zone for removal.
Conduction through a wall[]
These technologies transfer heat to the oil shale by conducting it through the retort wall. The shale feed usually consists of fine particles. Their advantage lies in the fact that retort vapors are not combined with combustion exhaust. The Combustion Resources process uses a hydrogen–fired rotating kiln, where hot gas is circulated through an outer annulus. The Oil-Tech staged electrically heated retort consists of individual inter-connected heating chambers, stacked atop each other. Its principal advantage lies in its modular design, which enhances its portability and adaptability. The Red Leaf Resources EcoShale In-Capsule Process combines surface mining with a lower-temperature heating method similar to in situ processes by operating within the confines of an earthen structure. A hot gas circulated through parallel pipes heats the oil shale rubble. An installation within the empty space created by mining would permit rapid reclamation of the topography. A general drawback of conduction through a wall technologies is that the retorts are more costly when scaled-up due to the resulting large amount of heat conducting walls made of high-temperature alloys.
Externally generated hot gas[]
In general, externally generated hot gas technologies are similar to internal combustion technologies in that they also process oil shale lumps in vertical shaft kilns. Significantly, though, the heat in these technologies is delivered by gases heated outside the retort vessel, and therefore the retort vapors are not diluted with combustion exhaust. The Petrosix and Paraho Indirect employ this technology. In addition to not accepting fine particles as feed, these technologies do not utilize the potential heat of combusting the char on the spent shale and thus must burn more valuable fuels. However, due to the lack of combustion of the spent shale, the oil shale does not exceed 500 °C (932 °F) and significant carbonate mineral decomposition and subsequent CO2 generation can be avoided for some oil shales. Also, these technologies tend to be the more stable and easier to control than internal combustion or hot solid recycle technologies.
Reactive fluids[]
Kerogen is tightly bound to the shale and resists dissolution by most solvents. Despite this constraint, extraction using especially reactive fluids has been tested, including those in a supercritical state. Reactive fluid technologies are suitable for processing oil shales with a low hydrogen content. In these technologies, hydrogen gas (H2) or hydrogen donors (chemicals that donate hydrogen during chemical reactions) react with coke precursors (chemical structures in the oil shale that are prone to form char during retorting but have not yet done so). Reactive fluid technologies include the IGT Hytort (high-pressure H2) process, donor solvent processes, and the Chattanooga fluidized bed reactor. In the IGT Hytort oil shale is processed in a high-pressure hydrogen environment. The Chattanooga process uses a fluidized bed reactor and an associated hydrogen-fired heater for oil shale thermal cracking and hydrogenation. Laboratory results indicate that these technologies can often obtain significantly higher oil yields than pyrolysis processes. Drawbacks are the additional cost and complexity of hydrogen production and high-pressure retort vessels.
Plasma gasification[]
Several experimental tests have been conducted for the oil-shale gasification by using plasma technologies. In these technologies, oil shale is bombarded by radicals (ions). The radicals crack kerogen molecules forming synthetic gas and oil. Air, hydrogen or nitrogen are used as plasma gas and processes may operate in an arc, plasma arc, or plasma electrolysis mode. The main benefit of these technologies is processing without using water.
In situ technologies[]
In situ technologies heat oil shale underground by injecting hot fluids into the rock formation, or by using linear or planar heating sources followed by thermal conduction and convection to distribute heat through the target area. Shale oil is then recovered through vertical wells drilled into the formation. These technologies are potentially able to extract more shale oil from a given area of land than conventional ex situ processing technologies, as the wells can reach greater depths than surface mines. They present an opportunity to recover shale oil from low-grade deposits that traditional mining techniques could not extract.
During World War II a modified in situ extraction process was implemented without significant success in Germany. One of the earliest successful in situ processes was underground gasification by electrical energy (Ljungström method)—a process exploited between 1940 and 1966 for shale oil extraction at Kvarntorp in Sweden. Prior to the 1980s, many variations of the in situ process were explored in the United States. The first modified in situ oil shale experiment in the United States was conducted by Occidental Petroleum in 1972 at Logan Wash, Colorado. Newer technologies are being explored that use a variety of heat sources and heat delivery systems.
Wall conduction[]
A simplified cross section of Shell's in situ process shows a number of vertical holes that have been drilled into the oil shale deposit, surrounded by a "freeze wall" intended to prevent leakage into the surrounding area. The process has an ecological footprint also on the ground. Shell's freeze wall for in situ shale oil production separates the process from its surroundings
Wall conduction in situ technologies use heating elements or heating pipes placed within the oil shale formation. The Shell in situ conversion process (Shell ICP) uses electrical heating elements for heating the oil shale layer to between 650 and 700 °F (340 and 370 °C) over a period of approximately four years. The processing area is isolated from surrounding groundwater by a freeze wall consisting of wells filled with a circulating super-chilled fluid. Disadvantages of this process are large electrical power consumption, extensive water use, and the risk of groundwater pollution. The process was tested since the early 1980s at the Mahogany test site in the Piceance Basin. 1,700 barrels (270 m3) of oil were extracted in 2004 at a 30-by-40-foot (9.1 by 12.2 m) testing area.
In the CCR Process proposed by American Shale Oil, superheated steam or another heat transfer medium is circulated through a series of pipes placed below the oil shale layer to be extracted. The system combines horizontal wells, through which steam is passed, and vertical wells, which provide both vertical heat transfer through refluxing of converted shale oil and a means to collect the produced hydrocarbons. Heat is supplied by combustion of natural gas or propane in the initial phase and by oil shale gas at a later stage.[10][49]
The Geothermic Fuels Cells Process (IEP GFC) proposed by Independent Energy Partners extracts shale oil by exploiting a high-temperature stack of fuel cells. The cells, placed in the oil shale formation, are fueled by natural gas during a warm-up period and afterward by oil shale gas generated by its own waste heat.
Externally generated hot gas[]
Schematic overview of the Chevron CRUSH process. Vertical wells inject hot gas, recover the oil, and house groundwater monitors. Oil pumps, hot gas compressors, and oil treatment units and tanks are located on the surface. The oil shale formation is fractured to enable gas circulation between wells and to increase oil recovery.
Chevron CRUSH process.[]
Externally generated hot gas in situ technologies use hot gases heated above-ground and then injected into the oil shale formation. The Chevron CRUSH process, which was researched by Chevron Corporation in partnership with Los Alamos National Laboratory, injects heated carbon dioxide into the formation via drilled wells and to heat the formation through a series of horizontal fractures through which the gas is circulated. General Synfuels International has proposed the Omnishale process involving injection of super-heated air into the oil shale formation. Mountain West Energy's In Situ Vapor Extraction process uses similar principles of injection of high-temperature gas.
ExxonMobil Electrofrac[]
Main article: ExxonMobil Electrofrac ExxonMobil's in situ technology (ExxonMobil Electrofrac) uses electrical heating with elements of both wall conduction and volumetric heating methods. It injects an electrically conductive material such as calcined petroleum coke into the hydraulic fractures created in the oil shale formation which then forms a heating element. Heating wells are placed in a parallel row with a second horizontal well intersecting them at their toe. This allows opposing electrical charges to be applied at either end.
Volumetric heating[]
An artist's cross section of an oil shale processing facility using radio waves to deliver heat to the formation. On a plateau surrounded by mountains, transmission towers, an oil derrick, and a few supporting structures are shown above ground. Large opaque pipes represent its underground infrastructure network . The Illinois Institute of Technology developed the concept of oil shale volumetric heating using radio waves (radio frequency processing) during the late 1970s. This technology was further developed by Lawrence Livermore National Laboratory. Oil shale is heated by vertical electrode arrays. Deeper volumes could be processed at slower heating rates by installations spaced at tens of meters. The concept presumes a radio frequency at which the skin depth is many tens of meters, thereby overcoming the thermal diffusion times needed for conductive heating. Its drawbacks include intensive electrical demand and the possibility that groundwater or char would absorb undue amounts of the energy. Radio frequency processing in conjunction with critical fluids is being developed by Raytheon together with CF Technologies and tested by Schlumberger.
Microwave heating technologies are based on the same principles as radio wave heating, although it is believed that radio wave heating is an improvement over microwave heating because its energy can penetrate farther into the oil shale formation. The microwave heating process was tested by Global Resource Corporation. Electro-Petroleum proposes electrically enhanced oil recovery by the passage of direct current between cathodes in producing wells and anodes located either at the surface or at depth in other wells. The passage of the current through the oil shale formation results in resistive Joule heating.
Oil sand extraction[]
Except for a fraction of the extra-heavy oil or bitumen which can be extracted by conventional oil well technology, oil sands must be produced by strip mining or the oil made to flow into wells using sophisticated in-situ techniques. These methods usually use more water and require larger amounts of energy than conventional oil extraction. While much of Canada's oil sands are being produced using open-pit mining, approximately 90% of Canadian oil sands and all of Venezuela's oil sands are too far below the surface to use surface mining.
Primary production[]
Conventional crude oil is normally extracted from the ground by drilling oil wells into a petroleum reservoir, allowing oil to flow into them under natural reservoir pressures, although artificial lift and techniques such as horizontal drilling, water flooding and gas injection are often required to maintain production. When primary production is used in the Venezuelan oil sands, where the extra-heavy oil is about 50 degrees Celsius, the typical oil recovery rates are about 8-12%. Canadian oil sands are much colder and more biodegraded, so bitumen recovery rates are usually only about 5-6%. Historically, primary recovery was used in the more fluid areas of Canadian oil sands. However, it recovered only a small fraction of the oil in place, so it not often used today.
Surface mining[]
Mining operations in the Athabasca oil sands. NASA Earth Observatory image, 2009. The Athabasca oil sands are the only major oil sands deposits which are shallow enough to surface mine. In the Athabasca sands there are very large amounts of bitumen covered by little overburden, making surface mining the most efficient method of extracting it. The overburden consists of water-laden muskeg (peat bog) over top of clay and barren sand. The oil sands themselves are typically 40 to 60 metres (130 to 200 ft) thick deposits of crude bitumen embedded in unconsolidated sandstone, sitting on top of flat limestone rock. Since Great Canadian Oil Sands (now Suncor Energy) started operation of the first large-scale oil sands mine in 1967, bitumen has been extracted on a commercial scale and the volume has grown at a steady rate ever since.
A large number of oil sands mines are currently in operation and more are in the stages of approval or development. The Syncrude Canada mine was the second to open in 1978, Shell Canada opened its Muskeg River mine (Albian Sands) in 2003 and Canadian Natural Resources Ltd (CNRL) opened its Horizon Oil Sands project in 2009. Newer mines include Shell Canada's Jackpine mine, Imperial Oil's Kearl Oil Sands Project, the Synenco Energy (now owned by Total S.A.) Northern Lights mine, and Suncor's Fort Hills mine.
Syncrude's Mildred Lake site, plant and tailings ponds Fort McMurray, Alberta Oil sands tailings ponds are engineered dam and dyke systems that contain salts, suspended solids and other dissolvable chemical compounds such as acids, benzene, hydrocarbons residual bitumen, fine silts (mature fine tails MFT), and water. Large volumes of tailings are a byproduct of surface mining of the oil sands and managing these tailings is one of the most difficult environmental challenges facing the oil sands industry. The Government of Alberta reported in 2013 that tailings ponds in the Alberta oil sands covered an area of about 77 square kilometres (30 sq mi). The Syncrude Tailings Dam or Mildred Lake Settling Basin (MLSB) is an embankment dam that is, by volume of construction material, the largest earth structure in the world in 2001.
Cold Heavy Oil Production with Sand (CHOPS)[]
Main article: Cold heavy oil production with sand Some years ago Canadian oil companies discovered that if they removed the sand filters from heavy oil wells and produced as much sand as possible with the oil, production rates improved significantly. This technique became known as Cold Heavy Oil Production with Sand (CHOPS). Further research disclosed that pumping out sand opened "wormholes" in the sand formation which allowed more oil to reach the wellbore. The advantage of this method is better production rates and recovery (around 10% versus 5-6% with sand filters in place) and the disadvantage that disposing of the produced sand is a problem. A novel way to do this was spreading it on rural roads, which rural governments liked because the oily sand reduced dust and the oil companies did their road maintenance for them. However, governments have become concerned about the large volume and composition of oil spread on roads; so in recent years disposing of oily sand in underground salt caverns has become more common.
Cyclic Steam Stimulation (CSS)[]
The use of steam injection to recover heavy oil has been in use in the oil fields of California since the 1950s. The cyclic steam stimulation (CSS) "huff-and-puff" method is now widely used in heavy oil production world-wide due to its quick early production rates; however recovery factors are relatively low (10-40% of oil in place) compared to SAGD (60-70% of OIP).
CSS has been in use by Imperial Oil at Cold Lake since 1985 and is also used by Canadian Natural Resources at Primrose and Wolf Lake and by Shell Canada at Peace River. In this method, the well is put through cycles of steam injection, soak, and oil production. First, steam is injected into a well at a temperature of 300 to 340 degrees Celsius for a period of weeks to months; then, the well is allowed to sit for days to weeks to allow heat to soak into the formation; and, later, the hot oil is pumped out of the well for a period of weeks or months. Once the production rate falls off, the well is put through another cycle of injection, soak and production. This process is repeated until the cost of injecting steam becomes higher than the money made from producing oil.
Steam Assisted Gravity Drainage (SAGD)[]
Steam assisted gravity drainage was developed in the 1980s by the Alberta Oil Sands Technology and Research Authority and fortuitously coincided with improvements in directional drilling technology that made it quick and inexpensive to do by the mid 1990s. In SAGD, two horizontal wells are drilled in the oil sands, one at the bottom of the formation and another about 5 metres above it. These wells are typically drilled in groups off central pads and can extend for miles in all directions. In each well pair, steam is injected into the upper well, the heat melts the bitumen, which allows it to flow into the lower well, where it is pumped to the surface.
SAGD has proved to be a major breakthrough in production technology since it is cheaper than CSS, allows very high oil production rates, and recovers up to 60% of the oil in place. Because of its economic feasibility and applicability to a vast area of oil sands, this method alone quadrupled North American oil reserves and allowed Canada to move to second place in world oil reserves after Saudi Arabia. Most major Canadian oil companies now have SAGD projects in production or under construction in Alberta's oil sands areas and in Wyoming. Examples include Japan Canada Oil Sands Ltd's (JACOS) project, Suncor's Firebag project, Nexen's Long Lake project, Suncor's (formerly Petro-Canada's) MacKay River project, Husky Energy's Tucker Lake and Sunrise projects, Shell Canada's Peace River project, Cenovus Energy's Foster Creek and Christina Lake developments, ConocoPhillips' Surmont project, Devon Canada's Jackfish project, and Derek Oil & Gas's LAK Ranch project. Alberta's OSUM Corp has combined proven underground mining technology with SAGD to enable higher recovery rates by running wells underground from within the oil sands deposit, thus also reducing energy requirements compared to traditional SAGD. This particular technology application is in its testing phase.
Vapor Extraction (VAPEX)[]
Several methods use solvents, instead of steam, to separate bitumen from sand. Some solvent extraction methods may work better in in situ production and other in mining. Solvent can be beneficial if it produces more oil while requiring less energy to produce steam.
Vapor Extraction Process (VAPEX) is an in situ technology, similar to SAGD. Instead of steam, hydrocarbon solvents are injected into an upper well to dilute bitumen and enables the diluted bitumen to flow into a lower well. It has the advantage of much better energy efficiency over steam injection, and it does some partial upgrading of bitumen to oil right in the formation. The process has attracted attention from oil companies, who are experimenting with it.
The above methods are not mutually exclusive. It is becoming common for wells to be put through one CSS injection-soak-production cycle to condition the formation prior to going to SAGD production, and companies are experimenting with combining VAPEX with SAGD to improve recovery rates and lower energy costs.
Toe to Heel Air Injection (THAI)[]
This is a very new and experimental method that combines a vertical air injection well with a horizontal production well. The process ignites oil in the reservoir and creates a vertical wall of fire moving from the "toe" of the horizontal well toward the "heel", which burns the heavier oil components and upgrades some of the heavy bitumen into lighter oil right in the formation. Historically fireflood projects have not worked out well because of difficulty in controlling the flame front and a propensity to set the producing wells on fire. However, some oil companies feel the THAI method will be more controllable and practical, and have the advantage of not requiring energy to create steam.
Advocates of this method of extraction state that it uses less freshwater, produces 50% less greenhouse gases, and has a smaller footprint than other production techniques.
Petrobank Energy and Resources has reported encouraging results from their test wells in Alberta, with production rates of up to 400 bbl/d (64 m3/d) per well, and the oil upgraded from 8 to 12 API degrees. The company hopes to get a further 7-degree upgrade from its CAPRI (controlled atmospheric pressure resin infusion) system, which pulls the oil through a catalyst lining the lower pipe.
After several years of production in situ, it has become clear that current THAI methods do not work as planned. Amid steady drops in production from their THAI wells at Kerrobert, Petrobank has written down the value of their THAI patents and the reserves at the facility to zero. They have plans to experiment with a new configuration they call "multi-THAI," involving adding more air injection wells.
Combustion Overhead Gravity Drainage (COGD)[]
This is an experimental method that employs a number of vertical air injection wells above a horizontal production well located at the base of the bitumen pay zone. An initial Steam Cycle similar to CSS is used to prepare the bitumen for ignition and mobility. Following that cycle, air is injected into the vertical wells, igniting the upper bitumen and mobilizing (through heating) the lower bitumen to flow into the production well. It is expected that COGD will result in water savings of 80% compared to SAGD.
heavy crude oil (or extra heavy crude oil) Extraction[]
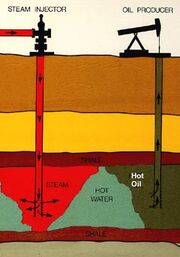
Steam is injected into many oil fields where the oil is thicker and heavier than normal crude oil.
Steam is injected into many oil fields where the oil is thicker and heavier than normal crude oil.
Production of heavy oil is becoming more common in many countries, with 2008 production led by Canada and Venezuela. Methods for extraction include Cold heavy oil production with sand, steam assisted gravity drainage, steam injection, vapor extraction, Toe-to-Heel Air Injection (THAI), and open-pit mining for extremely sandy and oil-rich deposits.
California thought it could lead to a major oil boom in the early 1980s, while both Canada and Venezuela thought of breaking the Arabs' hold on the oil market with it in the 1970s.
Fraking tight oil[]
Hydraulic fracturing (also hydrofracturing, hydrofracking, fracking, or fraccing) is a well-stimulation technique in which rock is fractured by a pressurized liquid. The process involves the high-pressure injection of 'fracking fluid' (primarily water, containing sand or other proppants suspended with the aid of thickening agents) into a wellbore to create cracks in the deep-rock formations through which natural gas, petroleum, and brine will flow more freely. When the hydraulic pressure is removed from the well, small grains of hydraulic fracturing proppants (either sand or aluminium oxide) hold the fractures open.
Hydraulic fracturing began as an experiment in 1947, and the first commercially successful application followed in 1950. As of 2012, 2.5 million "frac jobs" had been performed worldwide on oil and gas wells; over one million of those within the U.S. Such treatment is generally necessary to achieve adequate flow rates in shale gas, tight gas, tight oil, and coal seam gas wells. Some hydraulic fractures can form naturally in certain veins or dikes.
Hydraulic fracturing is highly controversial in many countries. Its proponents advocate the economic benefits of more extensively accessible hydrocarbons. However, opponents argue that these are outweighed by the potential environmental impacts, which include risks of ground and surface water contamination, air and noise pollution, and the triggering of earthquakes, along with the consequential hazards to public health and the environment.
Increases in seismic activity following hydraulic fracturing along dormant or previously unknown faults are sometimes caused by the deep-injection disposal of hydraulic fracturing flowback (a byproduct of hydraulically fractured wells), and produced formation brine (a byproduct of both fractured and nonfractured oil and gas wells). For these reasons, hydraulic fracturing is under international scrutiny, restricted in some countries, and banned altogether in others. Some countries have banned the practice or put moratoria in place, while others have adopted an approach involving tight regulation. The European Union is drafting regulations that would permit the controlled application of hydraulic fracturing.
Environmental issues[]

A Canadian oil sands tailings ponds.
There are many genuine concerns beyond the inevitable spoil heaps, slag heaps, tailings ponds, quarry pits, derelict facilities, flyash and mine-shafts. Some of the concerns are well known major issues, whilst others are vague rumors and new discoveries.
In their 2011 commissioned report entitled "Prudent Development: Realizing the Potential of North America’s Abundant Natural Gas and Oil Resources," the National Petroleum Council, an advisory committee to the U.S. Secretary of Energy, acknowledged health and safety concerns regarding the oil sands which include "volumes of water needed to generate issues of water sourcing; removal of overburden for surface mining can fragment wildlife habitat and increase the risk of soil erosion or surface run-off events to nearby water systems; GHG and other air emissions from production."
Oil sands extraction can badly affect the land when the bitumen is initially mined, water resources by its requirement for large quantities of water during separation of the oil and sand, and the air due to the release of carbon dioxide and other emissions. Heavy metals such as vanadium, nickel, lead, cobalt, mercury, chromium, cadmium, arsenic, selenium, copper, manganese, iron and zinc are naturally present in oil sands and may be concentrated by the extraction process. The environmental impact caused by oil sand extraction is frequently criticized by environmental groups such as Greenpeace, Climate Reality Project, Pembina Institute, 350.org, MoveOn.org, League of Conservation Voters, Patagonia, Sierra Club, and Energy Action Coalition. In particular, mercury contamination has been found around oil sands production in Alberta, Canada.
The European Union has indicated that it may vote to label oil sands oil as "highly polluting". Although oil sands exports to Europe are minimal, the issue has caused friction between the EU and Canada According to the California-based Jacobs Consultancy, the European Union used inaccurate and incomplete data in assigning a high greenhouse gas rating to gasoline derived from Alberta’s oilsands. Also, Iran, Saudi Arabia, Nigeria and Russia do not provide data on how much natural gas is released via flaring or venting in the oil extraction process. The Jacobs report pointed out that extra carbon emissions from oil-sand crude are 12 percent higher than from regular crude, although it was assigned a GHG rating 22% above the conventional benchmark by EU.
In 2014 results of a study published in the Proceedings of the National Academy of Sciences showed that official reports on emissions were not high enough. Report authors noted that, "emissions of organic substances with potential toxicity to humans and the environment are a major concern surrounding the rapid industrial development in the Athabasca oil sands region (AOSR)." This study found that tailings ponds were an indirect pathway transporting uncontrolled releases of evaporative emissions of three representative polycyclic aromatic hydrocarbon (PAH)s (phenanthrene, pyrene, and benzo(a)pyrene) and that these emissions had been previously unreported.
The physical and land environment[]
Land use and waste management[]
A large part of oil sands mining operations involves clearing trees and brush from a site and removing the overburden— topsoil, muskeg, sand, clay and gravel – that sits atop the oil sands deposit. Approximately 2.5 tons of oil sands are needed to produce one barrel of oil (roughly ⅛ of a ton). As a condition of licensing, projects are required to implement a reclamation plan. The mining industry asserts that the boreal forest will eventually colonize the reclaimed lands, but their operations are massive and work on long-term timeframes. As of 2013, about 715 square kilometres (276 sq mi) of land in the oil sands region have been disturbed, and 72 km2 (28 sq mi) of that land is under reclamation.
In March 2008, Alberta issued the first-ever oil sands land reclamation certificate to Syncrude for the 1.04 square kilometres (0.40 sq mi) parcel of land known as Gateway Hill approximately 35 kilometres (22 mi) north of Fort McMurray. Several reclamation certificate applications for oil sands projects are expected within the next 10 years.
Physical debris like ruble, etc[]
It is inevitable going to leave spoil heaps, ruble heaps, slag heaps, tailings ponds, quarry pits, derelict facilities, flyash and mine-shafts. There are post mining re-landscape and quarry refill\infill initiatives in most places in the Western World and most firms can and do obey this ideal. Such things became part of UK domestic law in the early1970s. Ireland also has simmilar laws.
Quarrying up large pine forests[]
Canada was accused with some basis in truth of planning to strip-mine forests and tundra in Alberta and eastern British Colombia in the early 2010s.
In the water, rivers and seas[]
General issues[]
More generally, waste oil, benzine and other chemicals can seep in to the water table and ruin it for man, beast and plants alike.
Water management[]
Between 2 and 4.5 volume units of water are used to produce each volume unit of synthetic crude oil in an ex-situ mining operation. According to Greenpeace, the Canadian oil sands operations use 349×106 m3/a (12.3×109 cu ft/a) of water, twice the amount of water used by the city of Calgary. However, in SAGD operations, 90–95% of the water is recycled and only about 0.2 volume units of water is used per volume unit of bitumen produced.
For the Athabasca oil sand operations water is supplied from the Athabasca River, the ninth longest river in Canada. The average flow just downstream of Fort McMurray is 633 m3/s (22,400 cu ft/s) with its highest daily average measuring 1,200 m3/s (42,000 cu ft/s). Oil sands industries water license allocations totals about 1.8% of the Athabasca river flow. Actual use in 2006 was about 0.4%. In addition, according to the Water Management Framework for the Lower Athabasca River, during periods of low river flow water consumption from the Athabasca River is limited to 1.3% of annual average flow.
In December 2010, the Oil Sands Advisory Panel, commissioned by former environment minister Jim Prentice, found that the system in place for monitoring water quality in the region, including work by the Regional Aquatic Monitoring Program, the Alberta Water Research Institute, the Cumulative Environmental Management Association and others, was piecemeal and should become more comprehensive and coordinated.
Water concerns become particularly sensitive issues in arid regions, such as the western US and Israel's Negev Desert, where plans exist to expand oil-shale extraction despite a water shortage.
Aquatic life deformities[]
There is conflicting research on the effects of the oil sands development on aquatic life. In 2007, Environment Canada completed a study that shows high deformity rates in fish embryos exposed to the oil sands. David W. Schindler, a limnologist from the University of Alberta, co-authored a study on Alberta's oil sands' contribution of aromatic polycyclic compounds, some of which are known carcinogens, to the Athabasca River and its tributaries. Scientists, local doctors, and residents supported a letter sent to the Prime Minister in September 2010 calling for an independent study of Lake Athabasca (which is downstream of the oil sands) to be initiated due to the rise of deformities and tumors found in fish caught there. The bulk of the research that defends the oil sands development is done by the Regional Aquatics Monitoring Program (RAMP). RAMP studies show that deformity rates are normal compared to historical data and the deformity rates in rivers upstream of the oil sands.
In the air and atmosphere[]
Air pollution management[]
The Alberta government computes an Air Quality Health Index (AQHI) from sensors in five communities in the oil sands region, operated by a "partner" called theWood Buffalo Environmental Association (WBEA). Each of their 17 continuously monitoring stations measure 3 to 10 air quality parameters among carbon monoxide(CO), hydrogen sulphide (H2S), total reduced sulfur (TRS), Ammonia (NH3), nitric oxide (NO), nitrogen dioxide (NO2), nitrogen oxides (NOx), ozone (O3), particulate matter (PM2.5), sulfur dioxide (SO2), total hydrocarbons (THC), and methane/non-methane hydrocarbons (CH4/NMHC). These AQHI are said to indicate 'low risk"'air quality more than 95% of the time. Prior to 2012, air monitoring showed significant increases in exceedances of hydrogen sulfide (H2S) both in the Fort McMurray area and near the oil sands upgraders. In 2007, the Alberta government issued an environmental protection order to Suncor in response to numerous occasions when ground level concentration for H2S) exceeded standards.
The Alberta Ambient Air Data Management System (AAADMS) of the Clean Air Strategic Alliance (aka CASA Data Warehouse) records that, during the year ending on 1 November 2015, there were 6 hourly reports of values exceeding the limit of 10 ppb for H2S, and 4 in 2013, down from 11 in 2014, and 73 in 2012. In September 2015, the Pembina Institute published a brief report about "a recent surge of odour and air quality concerns in northern Alberta associated with the expansion of oilsands development", contrasting the responses to these concerns in Peace River and Fort McKay. In Fort McKay, air quality is actively addressed by stakeholders represented in the WBEA, whereas the Peace River community must rely on the response of the Alberta Energy Regulator. In an effort to identify the sources of the noxious odours in the Fort McKay community, a Fort McKay Air Quality Index was established, extending the provincial Air Quality Health Index to include possible contributors to the problem: SO2, TRS, and THC. Despite these advantages, more progress was made in remediating the odour problems in the Peace River community, although only after some families had already abandoned their homes. The odour concerns in Fort McKay were reported to remain unresolved.
Greenhouse gas emissions[]
The production of bitumen and synthetic crude oil emits more greenhouse gases than the production of conventional crude oil. A 2009 study by the consulting firmIHS CERA estimated that production from Canada's oil sands emits "about 5% to 15% more carbon dioxide, over the "well-to-wheels" (WTW) lifetime analysis of the fuel, than average crude oil." Author and investigative journalist David Strahan that same year stated that IEA figures show that carbon dioxide emissions from the oil sands are 20% higher than average emissions from the petroleum production.
A Stanford University study commissioned by the EU in 2011 found that oil sands crude was as much as 22% more carbon intensive than other fuels. Greenpeace says the oil sands industry has been identified as the largest contributor to greenhouse gas emissions growth in Canada, as it accounts for 40 million tons of CO2 emissions per year. According to the Canadian Association of Petroleum Producers and Environment Canada the industrial activity undertaken to produce oil sands make up about 5% of Canada's greenhouse gas emissions, or 0.1% of global greenhouse gas emissions. It predicts the oil sands will grow to make up 8% of Canada's greenhouse gas emissions by 2015. While the production industrial activity emissions per barrel of bitumen produced decreased 26% over the decade 1992–2002, total emissions from production activity were expected to increase due to higher production levels. As of 2006, to produce one barrel of oil from the oil sands released almost 75 kilograms (165 lb) of greenhouse gases with total emissions estimated to be 67 megatonnes (66,000,000 long tons; 74,000,000 short tons) per year by 2015. A study by IHS CERA found that fuels made from Canadian oil sands resulted in significantly lower greenhouse gas emissions than many commonly cited estimates. A 2012 study by Swart and Weaver estimated that if only the economically viable reserve of 170 Gbbl (27×109 m3) oil sands was burnt, the global mean temperature would increase by 0.02 to 0.05 °C. If the entire oil-in-place of 1.8 trillion barrels were to be burnt, the predicted global mean temperature increase is 0.24 to 0.50 °C. Bergerson et al. found that while the WTW emissions can be higher than crude oil, the lower emitting oil sands cases can outperform higher emitting conventional crude cases.
To offset greenhouse gas emissions from the oil sands and elsewhere in Alberta, sequestering carbon dioxide emissions inside depleted oil and gas reservoirs has been proposed. This technology is inherited from enhanced oil recovery methods.
In July 2008, the Alberta government announced a C$2 billion fund to support sequestration projects in Alberta power plants and oil sands extraction and upgrading facilities. In November 2014, Fatih Birol, the chief economist of the International Energy Agency, described additional greenhouse gas emissions from Canada's oil sands as "extremely low". The IEA forecasts that in the next 25 years oil sands production in Canada will increase by more than 3 million barrels per day (480,000 m3/d), but Dr. Birol said "the emissions of this additional production is equal to only 23 hours of emissions of China — not even one day." The IEA is charged with responsibility for battling climate change, but Dr. Birol said he spends little time worrying about carbon emissions from oil sands. "There is a lot of discussion on oil sands projects in Canada and the United States and other parts of the world, but to be frank, the additional CO2 emissions coming from the oil sands is extremely low." Dr. Birol acknowledged that there is tremendous difference of opinion on the course of action regarding climate change, but added, "I hope all these reactions are based on scientific facts and sound analysis.". In 2014, the U.S. Congressional Research Service published a report in preparation for the decision about permitting construction of the Keystone XL pipeline. The report states in part: "Canadian oil sands crudes are generally more GHG emission-intensive than other crudes they may displace in U.S. refineries, and emit an estimated 17% more GHGs on a life-cycle basis than the average barrel of crude oil refined in the United States".
Carbon dioxide (CO2) released by it's physical burning[]
The burning of fossil fuels will always lead to the release of various amounts of soot, ash, carbon dioxide and greenhouse gas when burnt!
Public health impact[]
In 2007, it was suggested that wildlife has been negatively affected by the oil sands; for instance, moose were found in a 2006 study to have as high as 453 times the acceptable levels of arsenic in their systems, though later studies lowered this to 17 to 33 times the acceptable level (although below international thresholds for consumption). Concerns have been raised concerning the negative impacts that the oil sands have on public health, including higher than normal rates of cancer among residents of Fort Chipewyan. However, John O'Connor, the doctor who initially reported the higher cancer rates and linked them to the oil sands development, was subsequently investigated by the Alberta College of Physicians and Surgeons. The College later reported that O'Connor's statements consisted of "mistruths, inaccuracies and unconfirmed information." In 2010, the Royal Society of Canada released a report stating that "there is currently no credible evidence of environmental contaminant exposures from oil sands reaching Fort Chipewyan at levels expected to cause elevated human cancer rates." In August 2011, the Alberta government initiated a provincial health study to examine whether a link exists between the higher rates of cancer and the oil sands emissions. In a report released in 2014, Alberta’s Chief Medical Officer of Health, Dr. James Talbot, stated that "There isn’t strong evidence for an association between any of these cancers and environmental exposure [to tar sands]." Rather, Talbot suggested that the cancer rates at Fort Chipewyan, which were slightly higher compared with the provincial average, were likely due to a combination of factors such as high rates of smoking, obesity, diabetes, and alcoholism as well as poor levels of vaccination."
[]
Water is mixed with sand and chemicals to create fracking fluid. Approximately 40,000 gallons of chemicals are used per fracturing. A typical fracture treatment uses between 3 and 12 additive chemicals. Although there may be unconventional fracturing fluids
- Typical chemical additives can include one or more of the following:
- Acids—hydrochloric acid or acetic acid is used in the pre-fracturing stage for cleaning the perforations and initiating fissure in the near-wellbore rock.
- Sodium chloride (salt)—delays breakdown of gel polymer chains.
- Polyacrylamide and other friction reducers decrease turbulence in fluid flow and pipe friction, thus allowing the pumps to pump at a higher rate without having greater pressure on the surface.
- Ethylene glycol—prevents formation of scale deposits in the pipe.
- Borate salts—used for maintaining fluid viscosity during the temperature increase.
- Sodium and potassium carbonates—used for maintaining effectiveness of crosslinkers.
- Anaerobic, Biocide, BIO—Glutaraldehyde used as disinfectant of the water (bacteria elimination).
- Guar gum and other water-soluble gelling agents—increases viscosity of the fracturing fluid to deliver proppant into the formation more efficiently.
- Citric acid—used for corrosion prevention.
- Isopropanol—used to winterize the chemicals to ensure it doesn't freeze.[
- The most common chemical used for hydraulic fracturing in the United States in 2005–2009 was methanol, while some other most widely used chemicals were isopropyl alcohol, 2-butoxyethanol, and ethylene glycol.
- Typical fluid types are:-
- Conventional linear gels are cellulose derivative (carboxymethyl cellulose, hydroxyethyl cellulose, carboxymethyl hydroxyethyl cellulose, hydroxypropyl cellulose, hydroxyethyl methyl cellulose), guar or its derivatives (hydroxypropyl guar, carboxymethyl hydroxypropyl guar), mixed with other chemicals.[clarification needed]
- Borate-crosslinked fluids. These are guar-based fluids cross-linked with boron ions (from aqueous borax/boric acid solution). These gels have higher viscosity at pH 9 onwards and are used to carry proppant. After the fracturing job, the pH is reduced to 3–4 so that the cross-links are broken, and the gel is less viscous and can be pumped out.
- Organometallic-crosslinked fluids - zirconium, chromium, antimony, titanium salts - are known to crosslink guar-based gels. The crosslinking mechanism is not reversible, so once the proppant is pumped down along with cross-linked gel, the fracturing part is done. The gels are broken down with appropriate breakers.
- Aluminium phosphate-ester oil gels. Aluminium phosphate and ester oils are slurried to form cross-linked gel. These are one of the first known gelling systems.
- For slickwater fluids the use of sweeps is common. Sweeps are temporary reductions in the proppant concentration, which help ensure that the well is not overwhelmed with proppant. As the fracturing process proceeds, viscosity-reducing agents such as oxidizers and enzyme breakers are sometimes added to the fracturing fluid to deactivate the gelling agents and encourage flowback. Such oxidizer react with and break down the gel, reducing the fluid's viscosity and ensuring that no proppant is pulled from the formation. An enzyme acts as a catalyst for breaking down the gel. Sometimes pH modifiers are used to break down the crosslink at the end of a hydraulic fracturing job, since many require a pH buffer system to stay viscous. At the end of the job, the well is commonly flushed with water under pressure (sometimes blended with a friction reducing chemical.) Some (but not all) injected fluid is recovered. This fluid is managed by several methods, including underground injection control, treatment, discharge, recycling, and temporary storage in pits or containers. New technology is continually developing to better handle waste water and improve re-usability.
There is concern over the possible adverse public health implications of hydraulic fracturing activity. A 2013 review on shale gas production in the United States stated, "with increasing numbers of drilling sites, more people are at risk from accidents and exposure to harmful substances used at fractured wells." A 2011 hazard assessment recommended full disclosure of chemicals used for hydraulic fracturing and drilling as many have immediate health effects, and many may have long-term health effects.
In June 2014 Public Health England published a review of the potential public health impacts of exposures to chemical and radioactive pollutants as a result of shale gas extraction in the UK, based on the examination of literature and data from countries where hydraulic fracturing already occurs. The executive summary of the report stated:
- "An assessment of the currently available evidence indicates that the potential risks to public health from exposure to the emissions associated with shale gas extraction will be low if the operations are properly run and regulated. Most evidence suggests that contamination of groundwater, if it occurs, is most likely to be caused by leakage through the vertical borehole. Contamination of groundwater from the underground hydraulic fracturing process itself (ie the fracturing of the shale) is unlikely. However, surface spills of hydraulic fracturing fluids or wastewater may affect groundwater, and emissions to air also have the potential to impact on health. Where potential risks have been identified in the literature, the reported problems are typically a result of operational failure and a poor regulatory environment."
A 2012 report prepared for the European Union Directorate-General for the Environment identified potential risks to humans from air pollution and ground water contamination posed by hydraulic fracturing. This led to a series of recommendations in 2014 to mitigate these concerns. A 2012 guidance for pediatric nurses in the US said that hydraulic fracturing had a potential negative impact on public health and that pediatric nurses should be prepared to gather information on such topics so as to advocate for improved community health.
The potential environmental impacts of hydraulic fracturing include air emissions and climate change, high water consumption, water contamination, land use, risk of earthquakes, noise pollution, and health effects on humans. Air emissions are primarily methane that escapes from wells, along with industrial emissions from equipment used in the extraction process. Modern UK and EU regulation requires zero emissions of methane, a potent greenhouse gas. Escape of methane is a bigger problem in older wells than in ones built under more recent EU legislation.
Hydraulic fracturing uses between 1.2 and 3.5 million US gallons (4,500 and 13,200 m3) of water per well, with large projects using up to 5 million US gallons (19,000 m3). Additional water is used when wells are refractured. An average well requires 3 to 8 million US gallons (11,000 to 30,000 m3) of water over its lifetime. According to the Oxford Institute for Energy Studies, greater volumes of fracturing fluids are required in Europe, where the shale depths average 1.5 times greater than in the U.S. Surface water may be contaminated through spillage and improperly built and maintained waste pits, and ground water can be contaminated if the fluid is able to escape the formation being fractured (through, for example, abandoned wells) or by produced water (the returning fluids, which also contain dissolved constituents such as minerals and brine waters). Produced water is managed by underground injection, municipal and commercial wastewater treatment and discharge, self‐contained systems at well sites or fields, and recycling to fracture future wells. Typically less than half of the produced water used to fracture the formation is recovered.
About 3.6 hectares (8.9 acres) of land is needed per each drill pad for surface installations. Well pad and supporting structure construction significantly fragments landscapes which likely has negative effects on wildlife. These sites need to be remediated after wells are exhausted. Each well pad (in average 10 wells per pad) needs during preparatory and hydraulic fracturing process about 800 to 2,500 days of noisy activity, which affect both residents and local wildlife. In addition, noise is created by continuous truck traffic (sand, etc.) needed in hydraulic fracturing.
Research is underway to determine if human health has been affected by air and water pollution, and rigorous following of safety procedures and regulation is required to avoid harm and to manage the risk of accidents that could cause harm.
In July 2013, the US Federal Railroad Administration listed oil contamination by hydraulic fracturing chemicals as "a possible cause" of corrosion in oil tank cars.
Hydraulic fracturing sometimes causes induced seismicity or earthquakes. The magnitude of these events is usually too small to be detected at the surface, although tremors attributed to fluid injection into disposal wells have been large enough to have often been felt by people, and to have caused property damage and possibly injuries.
Microseismic events are often used to map the horizontal and vertical extent of the fracturing. A better understanding of the geology of the area being fracked and used for injection wells can be helpful in mitigating the potential for significant seismic events.
Lancastrian, Northumbrian and Sussexian earth tremors, burning Aussie lake gas bubbles, explosive gas in Wyoming tap water and stinky (most cases of methane, "human farts" and sulpher) are well known events of the early 2010s. Large parts of Wyoming are or were ruined by land blight and pollution of this kind.
Environmental impact of Heavy crude oil (or extra heavy crude oil)[]
With current production and transportation methods, heavy crudes have a more severe environmental impact than light ones. With more difficult production comes the employment of a variety of enhanced oil recovery techniques, including steam flooding and tighter well spacing, often as close as one well per acre. Heavy crude oils also carry contaminants. For example, Orinoco extra heavy oil contains 4.5% sulfur as well as vanadium and nickel. However, because crude oil is refined before use, generating specific alkanes via cracking and fractional distillation, this comparison is not valid in a practical sense. Heavy crude refining techniques may require more energy input though, so its environmental impact is presently more significant than that of lighter crude if the intended final products are light hydrocarbons (gasoline motor fuels). On the other hand, heavy crude is a better source for road asphalt mixes than light crude.
With present technology, the extraction and refining of heavy oils and oil sands generates as much as three times the total CO2 emissions compared to conventional oil, primarily driven by the extra energy consumption of the extraction process (which may include burning natural gas to heat and pressurize the reservoir to stimulate flow). Current research into better production methods seek to reduce this environmental impact.
In a 2009 report, the National Toxics Network, citing data provided by the Carbon Dioxide Information Analysis Center of the government of the United States and the Canadian Association of Petroleum Producers (CAPP), emissions of CO2 per unit of energy produced were ~84% of those for coal (0.078/0.093), higher than CO2 emissions of conventional oil.
Environmental Research Web has reported that "because of the energy needed for extraction and processing, petroleum from Canadian oil tar sands has higher life cycle emission" versus conventional fossil fuels; "up to 25% more."
Other environmental considerations[]
Conceren still serounds the environmental impact of the oil shale industry, since the mining oil shale involves a number of environmental impacts, more pronounced in surface mining than in underground mining. These include acid drainage induced by the sudden rapid exposure and subsequent oxidation of formerly buried materials, the introduction of metals including mercury into surface-water and groundwater, increased erosion, sulfur-gas emissions, and air pollution caused by the production of particulates during processing, transport, and support activities. In 2002, about 97% of air pollution, 86% of total waste and 23% of water pollution in Estonia came from the power industry, which uses oil shale as the main resource for its power production. Oil-shale extraction can damage the biological and recreational value of land and the ecosystem in the mining area. Combustion and thermal processing generate waste material.
In addition, the atmospheric emissions from oil shale processing and combustion include carbon dioxide, a greenhouse gas. Environmentalists oppose production and usage of oil shale, as it creates even more greenhouse gases than conventional fossil fuels. Section 526 of the Energy Independence And Security Act prohibits United States government agencies from buying oil produced by processes that produce more greenhouse gas emissions than would traditional petroleum. Experimental in situ conversion processes and carbon capture and storage technologies may reduce some of these concerns in the future, but at the same time they may cause other problems, including groundwater pollution. Among the water contaminants commonly associated with oil shale processing are oxygen and nitrogen heterocyclic hydrocarbons. Commonly detected examples include quinoline derivatives, pyridine, and various alkyl homologues of pyridine (picoline, lutidine). Some have expressed concerns over the oil shale industry's use of water. In 2002, the oil shale-fired power industry used 91% of the water consumed in Estonia. Depending on technology, above-ground retorting uses between one and five barrels of water per barrel of produced shale-oil.
A 2008 programmatic environmental impact statement issued by the US Bureau of Land Management stated that surface mining and retort operations produce 2 to 10 U.S. gallons (7.6 to 37.9 l; 1.7 to 8.3 imp gal) of waste water per 1 short ton (0.91 t) of processed oil shale. In situ processing, according to one estimate, uses about one-tenth as much water. Water concerns become particularly sensitive issues in arid regions, such as the western US and Israel's Negev Desert, where plans exist to expand oil-shale extraction despite a water shortage.
Environmental activists, including members of Greenpeace, have organized strong protests against the oil shale industry. In one result, Queensland Energy Resources put the proposed Stuart Oil Shale Project in Australia on hold in 2004.
Extraterrestrial oil shale[]
Some comets contain "massive amounts of an organic material almost identical to high grade oil shale," the equivalent of cubic kilometers of such mixed with other material; for instance, corresponding hydrocarbons were detected in a probe fly-by through the tail of Comet Halley during 1986. Some wonder wether this how organic matter fist got to Earth.
The meaning of the terms "Barrel of Oil Equivalent" (BOE) and "Barrel of Oil" (BO)[]
BOE is the acronym of Barrel of Oil Equivalent – a unit of energy based on the approximate energy released by burning one barrel of crude oil (BO).
- One BOE is roughly equivalent to 5,800 cf of natural gas or 58 CCF. The USGS takes a BOE for 6,000 cubic feet (170 cubic meters) of typical natural gas.
- One Barrel contains 42 US gallons or 158.9873 litres of light crude oil which will produce 5.8 × 106 British Thermal Units (million BTU or mBTU) according to the US Internal Revenue Service.
- 1 barrel (42 gallons) of crude oil = 5,800,000 Btu (for U.S. produced crude oil)
- 1 gallon of gasoline = 120,476 Btu
- 1 gallon of diesel fuel = 137,381 Btu (distillate fuel with less than 15 parts per million sulfur content)
- 1 gallon of heating oil = 138,500 Btu (distillate fuel with 15 to 500 parts per million sulfur content)
- 1 barrel of residual fuel oil = 6,287,000 Btu
- 1 cubic foot of natural gas = 1,028 Btu
- 1 gallon of propane = 91,333 Btu
- 1 short ton (2,000 pounds) of coal = 19,622,000 Btu
- 1 kilowatt-hour of electricity = 3,412 Btu
- 1 barrel (b) of petroleum or related products = 42 gallons
- 1 barrel of Portland cement = 376 pounds
- 1 barrel of flour = 196 pounds
- 1 barrel of pork or fish = 200 pounds
- 1 barrel of (U.S.) dry measure = 3.29122 bushels or 4.2104 cubic feet
- A barrel may be called a "drum," but a drum usually holds 55 gallons.
- Barrels or gallons for petroleum and alike (such as gasoline, diesel fuel, and jet fuel)
- Cubic feet for natural gas
- Tons for coal
- Kilowatthours for electricity
- Bushels for cereals and alike (such as rye, wheat and barley)
Note that all wights and thermal values are all set to pan-global averages since oil is not a uniform constant due to the natural global variations (light crude, medium crude, heavy crude, super-heavy crude, etc,), so some cases are inevitably going to be different.
Also see[]
- 1970s energy crises
- Atomic power stations
- Energy
- Time line of Iraq
- Estonian oil shale industry
- Narva Power Plant
- Narva Oil Plant
- Middle East
- OPEC
- Mining
- Minerals and fuel in central Africa
- Mineral mining, smelting and shipping videos
- Mid 1970s to early 1980s lean-burn power station technology
- Iranian Revolution
- Gulf Cooperation Council
- Cooperation Council for the Arab States of the Gulf
- Will nickel, oil, uranium and copper ever run out?
Links[]
- http://science.howstuffworks.com/oil-refining5.htm
- http://canadaconnects.ca/chemistry/10105/
- http://www.shukhov.org/shukhov.html
- https://en.wikipedia.org/wiki/Coal_oil
- https://en.wikipedia.org/wiki/Oil_shale_in_Estonia
- https://en.wikipedia.org/wiki/Kimmeridge_Oil_Field
- https://en.wikipedia.org/wiki/Wytch_Farm
- https://en.wikipedia.org/wiki/Kimmeridge
- https://en.wikipedia.org/wiki/Wytch_Farm
- https://en.wikipedia.org/wiki/Tight_oil
- http://www.heraldsun.com.au/news/national/trillion-shale-oil-find-surrounding-coober-pedy-can-fuel-australia/story-fndo471r-1226560401043
- http://www.news.com.au/business/companies/trillion-shale-oil-find-surrounding-coober-pedy-can-fuel-australia/story-fnda1bsz-1226560401043
- https://en.wikipedia.org/wiki/Countries_by_tight_oil_reserves
- http://www.dawn.com/news/1220955
- http://www.abc.net.au/news/2016-08-26/tight-oil-graph/7789400
- http://www.csur.com/sites/default/files/Understanding_TightOil_FINAL.pdf
- http://encyclopedia2.thefreedictionary.com/natural+asphalt
- https://en.wikipedia.org/wiki/Asphalt
- https://en.wikipedia.org/wiki/Pitch_Lake
- https://en.wikipedia.org/wiki/Asphalt
- https://en.wikipedia.org/wiki/La_Brea_Tar_Pits
- http://www.tarpits.org/
- http://www.quazoo.com/q/montan_wax
- https://en.wikipedia.org/wiki/Montan_wax
- http://www.strohmeyer.com/montan.htm
- https://en.wikipedia.org/wiki/Wax
- http://strohmeyer.com/montanwax.html
- https://images.search.yahoo.com/search/images;_ylt=A0LEV1f3bmlYcxgAeHtXNyoA;_ylu=X3oDMTEyNGQ0dWk3BGNvbG8DYmYxBHBvcwMxBHZ0aWQDQjMwMDlfMQRzZWMDc2M-?p=Montan+Wax&fr=yset_chr_cnewtab
- https://images.search.yahoo.com/search/images?p=lignite&fr=yset_chr_cnewtab&imgurl=http%3A%2F%2Fwww.caer.uky.edu%2Fkyasheducation%2Fimages%2Fccbs%2FLignite600.jpg#id=101&iurl=http%3A%2F%2Fwww.traderscity.com%2Fboard%2Fuserpix15%2F15095-grude-montan-wax-lignite-1.jpg&action=close
- http://voelpker.com/en/produkte/montan-waxes/montan-waxes.html
- https://www.merriam-webster.com/dictionary/montan%20wax
- http://www.eia.doe.gov/cneaf/coal/st_coal_pdf/0576h.pdf
- https://en.wikipedia.org/wiki/Nicolaus_von_Amsdorf
- https://gr.boell.org/en/lignite-greek-energy-system
- https://en.wikipedia.org/wiki/Abraham_Pineo_Gesner
- https://ar.wikipedia.org/wiki/%D8%A3%D9%85%D8%A8%D9%8A%D9%84%D8%A7%D9%8A%D8%AA
- http://scotsrootsresearch.com/pages/Articles-Shale%20Mining.html#sthash.ZwmGWEoL.dpuf
- https://en.wikipedia.org/wiki/Torbanite
- https://en.wikipedia.org/wiki/Oil_shale_geology
- https://en.wikipedia.org/wiki/Tasmanite
- https://en.wikipedia.org/wiki/Marinite
- https://en.wikipedia.org/wiki/Lamosite
- https://en.wikipedia.org/wiki/Kukersite
- https://en.wikipedia.org/w/index.php?title=Ampelite&oldid=475481170
- https://fr.wikipedia.org/wiki/Amp%C3%A9lite
- https://ar.wikipedia.org/wiki/%D8%A3%D9%85%D8%A8%D9%8A%D9%84%D8%A7%D9%8A%D8%AA
- http://pubs.usgs.gov/bul/0659/report.pdf
- http://pubs.usgs.gov/sir/2005/5294/pdf/sir5294_508.pdf
- https://en.wikipedia.org/wiki/Torbanite
- https://en.wikipedia.org/wiki/Coal_oil
- https://books.google.co.uk/books?id=QFemJs_jlvcC&pg=PA492&lpg=PA490&redir_esc=y#v=onepage&q&f=false
- http://www.scottishshale.co.uk/HistoryPages/index.html
- http://www.eccos.us/?option=com_content&view=article&id=17&Itemid=13
- http://subscribe.energyandcapital.com/99295
- http://index.about.com/index?qsrc=999&qo=semQuery&ad=semD&o=6018&l=sem&askid=aa7ba82b-748a-4a8c-8a94-cd71843d2566-0-ab_msb&q=shale%20oil%20shale%20gas&dqi=oil%20shale%20lothian&am=broad&an=msn_s
- http://www.idealprice.co.uk/compare.html?q=Oil-Shales-Sale&ort=Oil-Shales-Sale&adid=iaCkp56xzJ1fhsnDz5alWdDNoICbn461y5KemNSI1m6ZlNbViZSalNOf0g%3D%3D&baa=O&utm_source=bing&utm_medium=cpc&utm_campaign=O_1&utm_term=%2BOil%20%2BShales&utm_content=Oil%20Shales
- https://en.wikipedia.org/wiki/Coal_oil
- https://www.thebalance.com/us-shale-oil-boom-and-bust-3305553?utm_term=shale+oil+shale+gas&utm_content=p1-main-2-title&utm_medium=sem&utm_source=msn_s&utm_campaign=adid-aa7ba82b-748a-4a8c-8a94-cd71843d2566-0-ab_msb_ocode-6018&ad=semD&an=msn_s&am=broad&q=shale+oil+shale+gas&o=6018&qsrc=999&l=sem&askid=aa7ba82b-748a-4a8c-8a94-cd71843d2566-0-ab_msb
- https://www.thebalance.com/what-is-shale-oil-and-how-is-it-produced-3306195?utm_term=shale+oil+shale+gas&utm_content=p1-main-1-title&utm_medium=sem&utm_source=msn_s&utm_campaign=adid-aa7ba82b-748a-4a8c-8a94-cd71843d2566-0-ab_msb_ocode-6018&ad=semD&an=msn_s&am=broad&q=shale+oil+shale+gas&o=6018&qsrc=999&l=sem&askid=aa7ba82b-748a-4a8c-8a94-cd71843d2566-0-ab_msb
- http://subscribe.energyandcapital.com/99295
- https://en.wikipedia.org/wiki/Livingston,_West_Lothian#Livingston_Development_Corporation
- https://en.wikipedia.org/wiki/Spoil_tip
- https://en.wikipedia.org/wiki/Livingston_Village
- http://nora.nerc.ac.uk/11050/
- http://www.scottishshale.co.uk/
- http://www.geos.ed.ac.uk/homes/harvieb/bing.html
- http://www.geos.ed.ac.uk/homes/harvieb/bing.html
- http://scotsrootsresearch.com/pages/Articles-Shale%20Mining.html
- https://en.wikipedia.org/wiki/Oil_shale_industry
- https://en.wikipedia.org/wiki/James_Young_(chemist)
- https://en.wikipedia.org/wiki/History_of_the_oil_shale_industry
- https://en.wikipedia.org/wiki/Sangruntau_oil_shale_deposit
- https://en.wikipedia.org/wiki/Uzbekneftegaz
- https://en.wikipedia.org/wiki/History_of_the_oil_shale_industry_in_the_United_States
- https://en.wikipedia.org/wiki/Cracking_(chemistry)
- https://en.wikipedia.org/wiki/Heavy_crude_oil
- https://en.wikipedia.org/wiki/Steam_reforming
- http://www.cpchem.com/en-us/pages/default.aspx?Redirect=1
- http://www.wri.org/publication/content/10339
- http://www.eia.gov/emeu/finance/usi&to/upstream/venezuela.html
- http://earthscience.rice.edu%2Chttp//earthscience.rice.edu
- http://www.cpchem.com/en-us/pages/default.aspx?Redirect=1
- http://www.energy.alberta.ca/OilSands/pdfs/RPT_Chops_app3.pdf
- http://www.laohamutuk.org/Oil/Power/NTNHeavyOilMar09.pdf
- http://blog.environmentalresearchweb.org/2010/07/19/co2-emissions-from-tar-sands-a/
- http://cce.cornell.edu/EnergyClimateChange/NaturalGasDev/Documents/PDFs/fracking%20chemicals%20from%20a%20public%20health%20perspective.pdf
- https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/277219/Air.pdf
- http://energy.gov/sites/prod/files/2013/03/f0/ShaleGasPrimer_Online_4-2009.pdf
- https://en.wikipedia.org/wiki/Hydraulic_fracturing
- https://en.wikipedia.org/wiki/Directional_drilling
- https://en.wikipedia.org/wiki/Hydraulic_fracturing_by_country
- https://en.wikipedia.org/wiki/Frackman
- https://en.wikipedia.org/wiki/In_situ_leach
- https://en.wikipedia.org/wiki/Stranded_asset
- http://www.wgal.com/news/harrisburg-promise/41249616
- https://en.wikipedia.org/wiki/Asphaltene
- https://en.wikipedia.org/wiki/Gilsonite
- http://zista.co/en/products/gilsonite
- http://www.justthedesign.com/this-is-how-you-should-wear-the-off-the-shoulder-trend/
- https://web.archive.org/web/20100131053406/http://www.gstt.org/Geology/pitch%20lake.htm
- https://www.sciencenewsforstudents.org/
- https://en.wikipedia.org/wiki/Tar_pit
- https://www.nrdc.org/stories/dirty-fight-over-canadian-tar-sands-oil
- http://www.tarsandsworld.com/canada-oil-shale
- http://www.tarsandsworld.com/us-oil-shale
- http://ostseis.anl.gov/guide/tarsands/
- https://en.wikipedia.org/wiki/Oil_sands
- http://www.scottishshale.co.uk/index.html
- http://www.scottishshale.co.uk/KnowledgePages/Companies/index.html
- http://www.bbc.co.uk/news/uk-scotland-28085418
- http://www.neofuel.com/zuppero-1995-water-ice-nearly-everywhere-114647.pdf
- http://www.deseretnews.com/article/695263708/Oil-shale-rush-is-sparking-concern.html
- http://www.ceri-mines.org/documents/27symposium/presentations/av02-1qian.pdf
- http://www.aqua-calc.com/what-is/volume/oil-barrel
- http://www.petroleumhistory.org/OilHistory/pages/Barrels/barrels.html
- http://www.eia.gov/energyexplained/?page=about_energy_units
- http://whc.unesco.org/pg.cfm?cid=31&id_site=1029
- http://www.visit-dorset.com/things-to-do/attractions/kimmeridge-bay-p581223
- https://en.wikipedia.org/wiki/Kimmeridge_Oil_Field
- https://en.wikipedia.org/wiki/Shale_oil_extraction
- http://www.southampton.ac.uk/~imw/kimfire.htm
- http://www.scottishshale.co.uk/GazBeyond/BSEnglandShale/BSES_Dorset.html
- http://www.dorsetgeologistsassociation.com/RWG/oil-shales/KClshales.html
- https://en.wikipedia.org/wiki/Syncrude
- http://www.syncrude.ca/
- http://www.kimmeridgeenergy.com/
- http://www.ukogplc.com/ul/Kimmeridge%20Limestone%20Oil%20-%20The%20UK%20opportunity%20-%20Final%20Approved%20-%2015%20April%202016.pdf
- http://www.kimmeridgeenergy.com/about-us/our-team/
- http://www.kimmeridgeenergy.com/about-us/
- http://www.southampton.ac.uk/~imw/Kimmeridge-Oil-Shale.htm
- https://en.wikipedia.org/wiki/Kimmeridge
- https://simple.wikipedia.org/wiki/Kimmeridge_clay
- https://en.wikipedia.org/wiki/Kimmeridge_Oil_Field
- https://en.wikipedia.org/wiki/Oil_sands_tailings_ponds
- http://whc.unesco.org/pg.cfm?cid=31&id_site=1029
- https://en.wikipedia.org/wiki/Oil_sands
- http://www.2b1stconsulting.com/boe/
- http://oilsandstruth.org/russian-tar-sands
- http://www.capp.ca/canadian-oil-and-natural-gas/oil-sands/what-are-oil-sands
- http://priceofoil.org/campaigns/extreme-fossil-fuels/no-extreme-fossil-fuels-tar-sands/
- http://business.financialpost.com/news/energy/europe-softens-stance-on-canadas-oil-sands-as-relations-with-russia-sour
- http://www.statoil.com/en/about/worldwide/northamerica/canada/oilsands/pages/oilsands.aspx
- http://business.financialpost.com/news/energy/why-chinas-mood-is-souring-on-canadas-oil-patch?__lsa=b046-f4d6
- http://mainc.info/nth/og/pubs/prosp/chap5-eng.pdf
- http://pqasb.pqarchiver.com/csmonitor_historic/access/297535292.html?dids=297535292:297535292&FMT=ABS&FMTS=ABS:AI&date=Jul+27%2C+1938&author=By+D.M.+Edwards&pub=Christian+Science+Monitor&desc=Treasure+of+the+Arctic&pqatl=google
- http://www.geohelp.net/history.html
- http://www.albertasource.ca/petroleum/industry/historic_dev_canada_arctic.html
- http://www.canada.com/vancouversun/news/editorial/story.html?id=9d5b3935-8689-4314-b437-2299f6526bcc
- http://www.canada.com/saskatoonstarphoenix/news/national/story.html?id=dd5b3820-6b22-43a4-81a2-0d7620067a5e
- http://ostseis.anl.gov/guide/maps/index.cfm http://ostseis.anl.gov/documents/maps/OSTS002_UtahTarSands.pdf
- http://ostseis.anl.gov/guide/tarsands/index.cfm
- http://cat.inist.fr/?aModele=afficheN&cpsidt=1708232
- http://cat.inist.fr/?aModele=afficheN&cpsidt=1708232
- http://www.nevtahoilsands.com/the-utah-tar-sands.htm The Utah Tar Sands.
- http://findarticles.com/p/articles/mi_m5CNK/is_2007_Jan_12/ai_n24998908
- https://www.flickr.com/photos/boobook48/8231343515
- https://www.flickr.com/photos/boobook48/8231344141?ytcheck=1
- http://www.avalanchepress.com/ManchurianOil.php
- http://www.bloomberg.com/news/articles/2013-02-28/sunshine-oilsands-taps-china-for-output-boost-corporate-canada
- https://www.amazon.com/Fragrance-Oil-Reminiscence-jasmine-mandarin/dp/B00D9DEWUE?ie=UTF8&ascsubtag=shopzilla_mp_1199-20%3B14693722069003189705010090302008005&creative=395105&creativeASIN=B00D9DEWUE&linkCode=df0&ref_=asc_df_B00D9DEWUE4348016&smid=A3A63D76HKPKJO&tag=shopz0d-20
- http://www.scmp.com/business/commodities/article/1387098/chinas-oil-sands-bet-goes-sour-canada
- http://www.huffingtonpost.com/nicholas-ostroy/albertas-oil-sands-china-_b_1121471.html
- http://www.theglobeandmail.com/news/politics/chinas-oil-sands-deal-will-have-lasting-impact/article1357620/
- http://energychinaforum.com/news/88918.shtml
- http://www.hellenicshippingnews.com/wp-content/uploads/2015/05/china_oil_basin_map.png
- http://boereport.com/2013/10/25/north-america-leads-the-world-in-production-of-shale-gas/
- https://www.theguardian.com/environment/2012/feb/20/canada-eu-tar-sands
- http://www.nexencnoocltd.com/en/Operations/OilSands.aspx
- http://www.ccmresearch.co.uk/oil-sands.html
- http://www.no-tar-sands.org/
- http://www.zazzle.ca/welsh_oil_rig_trash_sticker_wales_oil_rigs_gas_round_sticker-217193030126997539
- https://www.theguardian.com/environment/2011/nov/27/canada-oil-sands-uk-backing
- http://www.ostseis.anl.gov/guide/tarsands/index.cfm
- http://www.bbc.co.uk/news/business-15889665
- http://www.rcinet.ca/en/2014/11/18/u-k-groups-protest-canadian-oilsands-lobbying/
- http://subscribe.energyandcapital.com/97584
- http://www.plus500.co.uk/Instruments/CL
- http://geology.com/articles/oil-sands/
- http://www.mining.com/eu-spares-canadian-oil-sands-the-dirty-fuel-label-93109/
- http://repository.icse.utah.edu/dspace/bitstream/123456789/6817/1/Utah-Tar-248.pdf
- http://berkshireoilinc.com/
- https://en.wikipedia.org/wiki/Oil_sands
- https://en.wikipedia.org/wiki/Utah_Oil_Sands_Joint_Venture
- http://ostseis.anl.gov/guide/tarsands/index.cfm
- https://en.wikipedia.org/wiki/Oil_sands
- https://en.wikipedia.org/wiki/Asphalt
- https://en.wikipedia.org/wiki/Synthetic_crude
- https://www.youtube.com/watch?v=86NG0j0wi1s
- https://en.wikipedia.org/wiki/Athabasca_oil_sands
- https://en.wikipedia.org/wiki/Oil_sands
- https://en.wikipedia.org/wiki/Oil_sands
- http://www.2b1stconsulting.com/boe/
- http://ostseis.anl.gov/guide/tarsands/
- http://www.oilsandsfactcheck.org/what-are-oil-sands/
- http://jobs.bakkenshale.com/
- https://en.wikipedia.org/wiki/Ploie%C8%99ti#Economy
- https://en.wikipedia.org/wiki/Operation_Tidal_Wave
- http://www.osti.gov/scitech/biblio/6567632
- http://www.southampton.ac.uk/~imw/Kimmeridge-Oil-Shale.htm
- https://www.geolsoc.org.uk/Groups-and-Networks/Specialist-Groups/History-of-Geology-Group/~/media/shared/documents/specialist%20and%20regional%20groups/hogg/hogg_weymouth%20abstract%20book.ashx
- http://one-potato-two-potato.blogspot.co.uk/
- http://www.hubbertpeak.com/laherrere/oilshalereview200509.pdf
- https://web.archive.org/web/20080512122018re_/encarta.msn.com/encyclopedia_761576221/petroleum.html
- http://www.sdnp.jo/International_Oil_Conference/rtos-A106.pdf
- https://e-reports-ext.llnl.gov/pdf/243505.pdf
- http://www.sfgate.com/news/article/Coaxing-oil-from-huge-U-S-shale-deposits-2489359.php
- https://fas.org/sgp/crs/misc/RL33359.pdf
- http://www.blm.gov/wo/st/en/info/newsroom/2005/september/NR_050920.html
- http://ostseis.anl.gov/eis/what/index.cfm
- https://fr.wikipedia.org/wiki/Mine_des_Télots
- https://en.wikipedia.org/wiki/Building-integrated_photovoltaics
- http://www.solarserver.com/solar-magazine/solar-report/solar-report/building-integrated-photovoltaics-an-emerging-market.html
- This article incorporates text from a publication now in the public domain: Chambers, Ephraim, ed. (1728). "article name needed". Cyclopædia, or an Universal Dictionary of Arts and Sciences (first ed.). James and John Knapton, et al. It also contains public domain content from the 1751 edition